The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial
- PMID: 38258024
- PMCID: PMC10819176
- DOI: 10.3390/microorganisms12010198
The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial
Abstract
Background: Antibiotic-associated diarrhea is a condition reported in 5-35% of patients treated with antibiotics, especially in older patients with comorbidities. In most cases, antibiotic-associated diarrhea is not associated with serious complications, but it can prolong hospitalization and provoke Clostridium difficile infection. An important role in the prevention of antibiotic-associated diarrhea is carried out by some probiotic strains such as Lactobacillus GG or the yeast Saccharomyces boulardii that showed good efficacy and a significant reduction in antibiotic-associated diarrhea. Similarly, the Limosilactobacillus reuteri DSM 17938 showed significant benefits in acute diarrhea, reducing its duration and abdominal pain.
Aim: The aim of this study was to test the efficacy of a mix of two probiotic strains (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103; Reuterin GG®, NOOS, Italy), in association with antibiotics (compared to antibiotics used alone), in reducing antibiotic-associated diarrhea, clostridium difficile infection, and other gastrointestinal symptoms in adult hospitalized patients.
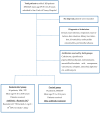
Patients and methods: We enrolled 113 (49M/64F, mean age 69.58 ± 21.28 years) adult patients treated with antibiotics who were hospitalized at the Internal Medicine Department of the San Carlo di Nancy Hospital in Rome from January 2023 to September 2023. Patients were randomized to receive probiotics 1.4 g twice/day in addition with antibiotics (Reuterin GG® group, total: 56 patients, 37F/19M, 67.16 ± 20.5 years old) or antibiotics only (control group, total: 57 patients, 27F/30 M, 71 ± 22 years old).
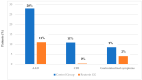
Results: Patients treated with Reuterin GG® showed a significant reduction in diarrhea and clostridium difficile infection. In particular, 28% (16/57) of patients in the control group presented with diarrhea during treatment, compared with 11% (6/56) in the probiotic group (p < 0.05). Interestingly, 7/57 (11%) of patients treated only with antibiotics developed clostridium difficile infection compared to 0% in the probiotic group (p < 0.01). Finally, 9% (5/57) of patients in the control group presented with vomiting compared with 2% (1/56) in the probiotic group (p < 0.05).
Conclusions: Our study showed, for the first time, the efficacy of these two specific probiotic strains in preventing antibiotic-associated diarrhea and clostridium difficile infection in adult hospitalized patients treated with antibiotic therapy. This result allows us to hypothesize that the use of specific probiotic strains during antibiotic therapy can prevent dysbiosis and subsequent antibiotic-associated diarrhea and clostridium difficile infection, thus resulting in both patient and economic health care benefits.
Keywords: Lacticaseibacillus rhamnosus GG ATCC 53103; Limosilactobacillus reuteri LMG P-27481; antibiotic; diarrhea; probiotic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Doron S., Gorbach S.L. Probiotics: Their role in the treatment and prevention of disease. Expert. Rev. Anti Infect. Ther. 2006;4:261–275. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases