Oral Absorption of Middle-to-Large Molecules and Its Improvement, with a Focus on New Modality Drugs
- PMID: 38258058
- PMCID: PMC10820198
- DOI: 10.3390/pharmaceutics16010047
Oral Absorption of Middle-to-Large Molecules and Its Improvement, with a Focus on New Modality Drugs
Abstract
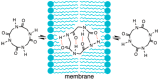
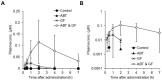
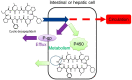
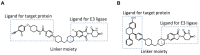
To meet unmet medical needs, middle-to-large molecules, including peptides and oligonucleotides, have emerged as new therapeutic modalities. Owing to their middle-to-large molecular sizes, middle-to-large molecules are not suitable for oral absorption, but there are high expectations around orally bioavailable macromolecular drugs, since oral administration is the most convenient dosing route. Therefore, extensive efforts have been made to create bioavailable middle-to-large molecules or develop absorption enhancement technology, from which some successes have recently been reported. For example, Rybelsus® tablets and Mycapssa® capsules, both of which contain absorption enhancers, were approved as oral medications for type 2 diabetes and acromegaly, respectively. The oral administration of Rybelsus and Mycapssa exposes their pharmacologically active peptides with molecular weights greater than 1000, namely, semaglutide and octreotide, respectively, into systemic circulation. Although these two medications represent major achievements in the development of orally absorbable peptide formulations, the oral bioavailability of peptides after taking Rybelsus and Mycapssa is still only around 1%. In this article, we review the approaches and recent advances of orally bioavailable middle-to-large molecules and discuss challenges for improving their oral absorption.
Keywords: C10; C8; Lipinski’s rule of five; SNAC; absorption enhancer; antisense oligonucleotide; cyclic peptide; middle-to-large molecule; new modality; target protein degrader.
Conflict of interest statement
All authors are the employees of Daiichi Sankyo Co., Ltd. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources

