Development of α-Cyclodextrin-Based Orally Disintegrating Tablets for 4-Phenylbutyrate
- PMID: 38258093
- PMCID: PMC10818935
- DOI: 10.3390/pharmaceutics16010082
Development of α-Cyclodextrin-Based Orally Disintegrating Tablets for 4-Phenylbutyrate
Abstract
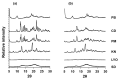
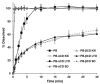
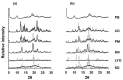
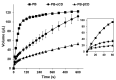
Despite major improvements brought about by the introduction of taste-masked formulations of 4-phenylbutyrate (PB), poor compliance remains a significant drawback to treatment for some pediatric and dysphagic patients with urea cycle disorders (UCDs). This study reports on the development of a cyclodextrin (CD)-based orally disintegrating tablet (ODT) formulation for PB as an alternative to existing formulations. This is based on previous reports of the PB taste-masking potential of CDs and the suitability of ODTs for improving compliance in pediatric and dysphagic populations. In preliminary studies, the interactions of PB with α and βCD in the solid state were characterized using X-ray diffraction, scanning electron microscopy, dissolution, and accelerated stability studies. Based on these studies, lyophilized PB-CD solid systems were formulated into ODTs after wet granulation. Evaluation of the ODTs showed that they had adequate physical characteristics, including hardness and friability and good storage stability. Notably, the developed αCD-based ODT for PB had a disintegration time of 28 s and achieved a slightly acidic and agreeable pH (≈5.5) in solution, which is suitable for effective PB-CD complexation and taste masking. The developed formulation could be helpful as an alternative to existing PB formulations, especially for pediatric and dysphagic UCD patients.
Keywords: 4-phenylbutyrate; cyclodextrins; orally disintegrating tablets; pediatric; solid-state complexation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources

