Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques
- PMID: 38258878
- PMCID: PMC10860463
- DOI: 10.1080/22221751.2024.2309985
Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques
Abstract
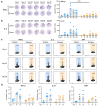
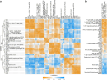
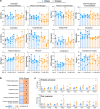
Herpes zoster remains an important global health issue and mainly occurs in aged and immunocompromised individuals with an early exposure history to Varicella Zoster Virus (VZV). Although the licensed vaccine Shingrix has remarkably high efficacy, undesired reactogenicity and increasing global demand causing vaccine shortage urged the development of improved or novel VZV vaccines. In this study, we developed a novel VZV mRNA vaccine candidate (named as ZOSAL) containing sequence-optimized mRNAs encoding full-length glycoprotein E encapsulated in an ionizable lipid nanoparticle. In mice and rhesus macaques, ZOSAL demonstrated superior immunogenicity and safety in multiple aspects over Shingrix, especially in the induction of strong T-cell immunity. Transcriptomic analysis revealed that both ZOSAL and Shingrix could robustly activate innate immune compartments, especially Type-I IFN signalling and antigen processing/presentation. Multivariate correlation analysis further identified several early factors of innate compartments that can predict the magnitude of T-cell responses, which further increased our understanding of the mode of action of two different VZV vaccine modalities. Collectively, our data demonstrated the superiority of VZV mRNA vaccine over licensed subunit vaccine. The mRNA platform therefore holds prospects for further investigations in next-generation VZV vaccine development.
Keywords: Shingrix; Varicella Zoster Virus; immune mechanisms; mRNA vaccine; nonhuman primate.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
