Schwann cells modulate nociception in neurofibromatosis 1
- PMID: 38258905
- PMCID: PMC10906222
- DOI: 10.1172/jci.insight.171275
Schwann cells modulate nociception in neurofibromatosis 1
Abstract
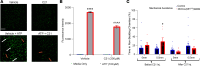
Pain of unknown etiology is frequent in individuals with the tumor predisposition syndrome neurofibromatosis 1 (NF1), even when tumors are absent. Nerve Schwann cells (SCs) were recently shown to play roles in nociceptive processing, and we find that chemogenetic activation of SCs is sufficient to induce afferent and behavioral mechanical hypersensitivity in wild-type mice. In mouse models, animals showed afferent and behavioral hypersensitivity when SCs, but not neurons, lacked Nf1. Importantly, hypersensitivity corresponded with SC-specific upregulation of mRNA encoding glial cell line-derived neurotrophic factor (GDNF), independently of the presence of tumors. Neuropathic pain-like behaviors in the NF1 mice were inhibited by either chemogenetic silencing of SC calcium or by systemic delivery of GDNF-targeting antibodies. Together, these findings suggest that alterations in SCs directly modulate mechanical pain and suggest cell-specific treatment strategies to ameliorate pain in individuals with NF1.
Keywords: Calcium; Mouse models; Neuroscience; Pain.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

