Metagenomic insights into the dynamic degradation of brown algal polysaccharides by kelp-associated microbiota
- PMID: 38259074
- PMCID: PMC10880675
- DOI: 10.1128/aem.02025-23
Metagenomic insights into the dynamic degradation of brown algal polysaccharides by kelp-associated microbiota
Abstract
Marine bacteria play important roles in the degradation and cycling of algal polysaccharides. However, the dynamics of epiphytic bacterial communities and their roles in algal polysaccharide degradation during kelp decay are still unclear. Here, we performed metagenomic analyses to investigate the identities and predicted metabolic abilities of epiphytic bacterial communities during the early and late decay stages of the kelp Saccharina japonica. During kelp decay, the dominant epiphytic bacterial communities shifted from Gammaproteobacteria to Verrucomicrobia and Bacteroidetes. In the early decay stage of S. japonica, epiphytic bacteria primarily targeted kelp-derived labile alginate for degradation, among which the gammaproteobacterial Vibrionaceae (particularly Vibrio) and Psychromonadaceae (particularly Psychromonas), abundant in alginate lyases belonging to the polysaccharide lyase (PL) families PL6, PL7, and PL17, were key alginate degraders. More complex fucoidan was preferred to be degraded in the late decay stage of S. japonica by epiphytic bacteria, predominantly from Verrucomicrobia (particularly Lentimonas), Pirellulaceae of Planctomycetes (particularly Rhodopirellula), Pontiellaceae of Kiritimatiellota, and Flavobacteriaceae of Bacteroidetes, which depended on using glycoside hydrolases (GHs) from the GH29, GH95, and GH141 families and sulfatases from the S1_15, S1_16, S1_17, and S1_25 families to depolymerize fucoidan. The pathways for algal polysaccharide degradation in dominant epiphytic bacterial groups were reconstructed based on analyses of metagenome-assembled genomes. This study sheds light on the roles of different epiphytic bacteria in the degradation of brown algal polysaccharides.IMPORTANCEKelps are important primary producers in coastal marine ecosystems. Polysaccharides, as major components of brown algal biomass, constitute a large fraction of organic carbon in the ocean. However, knowledge of the identities and pathways of epiphytic bacteria involved in the degradation process of brown algal polysaccharides during kelp decay is still elusive. Here, based on metagenomic analyses, the succession of epiphytic bacterial communities and their metabolic potential were investigated during the early and late decay stages of Saccharina japonica. Our study revealed a transition in algal polysaccharide-degrading bacteria during kelp decay, shifting from alginate-degrading Gammaproteobacteria to fucoidan-degrading Verrucomicrobia, Planctomycetes, Kiritimatiellota, and Bacteroidetes. A model for the dynamic degradation of algal cell wall polysaccharides, a complex organic carbon, by epiphytic microbiota during kelp decay was proposed. This study deepens our understanding of the role of epiphytic bacteria in marine algal carbon cycling as well as pathogen control in algal culture.
Keywords: alginate; epiphytic bacteria; fucoidan; host–microbe interaction; kelp; metagenomics; polysaccharide degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


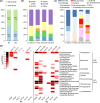


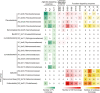



References
-
- Wiencke C, Amsler CD. 2012. Seaweeds and their communities in polar regions, p 265–291. In Wiencke C, Bischof K (ed), Seaweed biology: novel insights into ecophysiology, ecology and utilization. Vol. 219. Springer, Berlin, Germany.
-
- Krause-Jensen D, Duarte CM. 2016. Substantial role of macroalgae in marine carbon sequestration. Nature Geosci 9:737–742. doi: 10.1038/ngeo2790 - DOI
-
- Hardison AK, Canuel EA, Anderson IC, Veuger B. 2010. Fate of macroalgae in benthic systems: carbon and nitrogen cycling within the microbial community. Mar Ecol Prog Ser 414:41–55. doi: 10.3354/meps08720 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 42176229, 42376106/MOST | National Natural Science Foundation of China (NSFC)
- 32270047/MOST | National Natural Science Foundation of China (NSFC)
- 2022QNLM030004-3/Marine S&T Fund of Shandong Province for Qingdao Marine Science and Technology Center
- 2022YFC2807503/National Key R&D Program of China
- 2021CXGC010502/Key R&D Program of Shandong Province
LinkOut - more resources
Full Text Sources

