The mechanism of ceftazidime and cefiderocol hydrolysis by D179Y variants of KPC carbapenemases is similar and involves the formation of a long-lived covalent intermediate
- PMID: 38259088
- PMCID: PMC10916376
- DOI: 10.1128/aac.01108-23
The mechanism of ceftazidime and cefiderocol hydrolysis by D179Y variants of KPC carbapenemases is similar and involves the formation of a long-lived covalent intermediate
Abstract
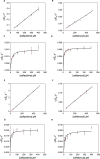
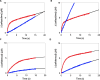
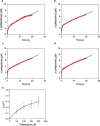
Klebsiella pneumoniae carbapenemase (KPC) variants have been described that confer resistance to both ceftazidime-avibactam and cefiderocol. Of these, KPC-33 and KPC-31 are D179Y-containing variants derived from KPC-2 and KPC-3, respectively. To better understand this atypical phenotype, the catalytic mechanism of ceftazidime and cefiderocol hydrolysis by KPC-33 and KPC-31 as well as the ancestral KPC-2 and KPC-3 enzymes was studied. Steady-state kinetics showed that the D179Y substitution in either KPC-2 or KPC-3 is associated with a large decrease in both kcat and KM such that kcat/KM values were largely unchanged for both ceftazidime and cefiderocol substrates. A decrease in both kcat and KM is consistent with a decreased and rate-limiting deacylation step. We explored this hypothesis by performing pre-steady-state kinetics and showed that the acylation step is rate-limiting for KPC-2 and KPC-3 for both ceftazidime and cefiderocol hydrolysis. In contrast, we observed a burst of acyl-enzyme formation followed by a slow steady-state rate for the D179Y variants of KPC-2 and KPC-3 with either ceftazidime or cefiderocol, indicating that deacylation of the covalent intermediate is the rate-limiting step for catalysis. Finally, we show that the low KM value for ceftazidime or cefiderocol hydrolysis of the D179Y variants is not an indication of tight binding affinity for the substrates but rather is a reflection of the deacylation reaction becoming rate-limiting. Thus, the hydrolysis mechanism of ceftazidime and cefiderocol by the D179Y variants is very similar and involves the formation of a long-lived covalent intermediate that is associated with resistance to the drugs.
Keywords: KPC-31 β-lactamase; KPC-33 β-lactamase; antibiotic resistance; cross-resistance; enzyme variants; transient kinetics; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi:10.1128/AAC.45.4.1151-1161.2001 - DOI - PMC - PubMed
-
- Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, et al. . 2019. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae. Clin Infect Dis 68:355–364. doi:10.1093/cid/ciy492 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

