Lung Fibroblasts Take up Breast Cancer Cell-derived Extracellular Vesicles Partially Through MEK2-dependent Macropinocytosis
- PMID: 38259097
- PMCID: PMC10802141
- DOI: 10.1158/2767-9764.CRC-23-0316
Lung Fibroblasts Take up Breast Cancer Cell-derived Extracellular Vesicles Partially Through MEK2-dependent Macropinocytosis
Abstract
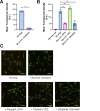
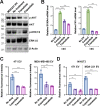
Extracellular vesicles (EV) have emerged as critical effectors in the cross-talk between cancer and normal cells by transferring intracellular materials between adjacent or distant cells. Previous studies have begun to elucidate how cancer cells, by secreting EVs, adapt normal cells at a metastatic site to facilitate cancer cell metastasis. In this study, we utilized a high-content microscopic screening platform to investigate the mechanisms of EV uptake by primary lung fibroblasts. A selected library containing 90 FDA-approved anticancer drugs was screened for the effect on fibroblast uptake of EVs from MDA-MB-231 breast cancer cells. Among the drugs identified to inhibit EV uptake without exerting significant cytotoxicity, we validated the dose-dependent effect of Trametinib (a MEK1/2 inhibitor) and Copanlisib (a PI3K inhibitor). Trametinib suppressed macropinocytosis in lung fibroblasts and inhibited EV uptake with a higher potency comparing with Copanlisib. Gene knockdown and overexpression studies demonstrated that uptake of MDA-MB-231 EVs by lung fibroblasts required MEK2. These findings provide important insights into the mechanisms underlying lung fibroblast uptake of breast cancer cell-derived EVs, which could play a role in breast cancer metastasis to the lungs and suggest potential therapeutic targets for preventing or treating this deadly disease.
Significance: Through a phenotypic screen, we found that MEK inhibitor Trametinib suppressed EV uptake and macropinocytosis in lung fibroblasts, and that EV uptake is mediated by MEK2 in these cells. Our results suggest that MEK2 inhibition could serve as a strategy to block cancer EV uptake by lung fibroblasts.
© 2024 The Authors; Published by the American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

