Optimizing percutaneous pulmonary valve implantation with patient-specific 3D-printed pulmonary artery models and hemodynamic assessment
- PMID: 38259310
- PMCID: PMC10800937
- DOI: 10.3389/fcvm.2023.1331206
Optimizing percutaneous pulmonary valve implantation with patient-specific 3D-printed pulmonary artery models and hemodynamic assessment
Abstract
Background: Percutaneous pulmonary valve implantation (PPVI) has emerged as a less invasive alternative for treating severe pulmonary regurgitation after tetralogy of Fallot (TOF) repair in patients with a native right ventricular outflow tract (RVOT). However, the success of PPVI depends on precise patient-specific valve sizing, the avoidance of oversizing complications, and optimal valve performance. In recent years, innovative adaptations of commercially available cardiovascular mock loops have been used to test conduits in the pulmonary position. These models are instrumental in facilitating accurate pulmonic valve sizing, mitigating the risk of oversizing, and providing insight into the valve performance before implantation. This study explored the utilization of custom-modified mock loops to implant patient-specific 3D-printed pulmonary artery geometries, thereby advancing PPVI planning and execution.
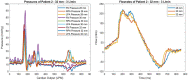
Material and methods: Patient-specific 3D-printed pulmonary artery geometries of five patients who underwent PPVI using Pulsta transcatheter heart valve (THV) ® were tested in a modified ViVitro pulse duplicator system®. Various valve sizes were subjected to 10 cycles of testing at different cardiac output levels. The transpulmonary systolic and regurgitation fractions of the valves were also recorded and compared.
Results: A total of 39 experiments were conducted using five different patient geometries and several different valve sizes (26, 28, 30, and 32 mm) at 3, 4, and 5 L/min cardiac output at heart rates of 70 beats per minute (bpm) and 60/40 systolic/diastolic ratios. The pressure gradients and regurgitation fractions of the tested valve sizes in the models were found to be similar to the pressure gradients and regurgitation fractions of valves used in real procedures. However, in two patients, different valve sizes showed better hemodynamic values than the actual implanted valves.
Discussion: The use of 3D printing technology, electromagnetic flow meters, and the custom-modified ViVitro pulse duplicator system® in conjunction with patient-specific pulmonary artery models has enabled a comprehensive assessment of percutaneous pulmonic valve implantation performance. This approach allows for accurate valve sizing, minimization of oversizing risks, and valuable insights into hemodynamic behavior before implantation. The data obtained from this experimental setup will contribute to advancing PPVI procedures and offer potential benefits in improving patient outcomes and safety.
Keywords: 3D models; Pulsta; ViVitro; ViVitro percutaneous pulmonary valve implantation; hemodynamic; in vitro hemodynamic; percutaneous pulmonary valve implantation; tetralogy of fallot.
© 2024 Odemis, AKA, Ali, Gumus and Pekkan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
3D Modeling of Self-Expandable Valves for PPVI in Distinct RVOT Morphologies.Pediatr Cardiol. 2025 Feb 9. doi: 10.1007/s00246-025-03796-7. Online ahead of print. Pediatr Cardiol. 2025. PMID: 39923207
-
Early Outcomes of Percutaneous Pulmonary Valve Implantation with Pulsta and Melody Valves: The First Report from Korea.J Clin Med. 2020 Aug 26;9(9):2769. doi: 10.3390/jcm9092769. J Clin Med. 2020. PMID: 32859019 Free PMC article.
-
The adaptability of the Pulsta valve to the diverse main pulmonary artery shape of native right ventricular outflow tract disease.Catheter Cardiovasc Interv. 2024 Mar;103(4):587-596. doi: 10.1002/ccd.30968. Epub 2024 Feb 11. Catheter Cardiovasc Interv. 2024. PMID: 38341624
-
Percutaneous Pulmonary Valve Implantation: Present Status and Evolving Future.J Am Coll Cardiol. 2015 Nov 17;66(20):2246-2255. doi: 10.1016/j.jacc.2015.09.055. J Am Coll Cardiol. 2015. PMID: 26564602 Review.
-
Percutaneous Pulmonary Valve Implantation.Korean Circ J. 2020 Apr;50(4):302-316. doi: 10.4070/kcj.2019.0291. Korean Circ J. 2020. PMID: 32157831 Free PMC article. Review.
Cited by
-
3D Modeling of Self-Expandable Valves for PPVI in Distinct RVOT Morphologies.Pediatr Cardiol. 2025 Feb 9. doi: 10.1007/s00246-025-03796-7. Online ahead of print. Pediatr Cardiol. 2025. PMID: 39923207
-
A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation.Bioengineering (Basel). 2025 Mar 26;12(4):344. doi: 10.3390/bioengineering12040344. Bioengineering (Basel). 2025. PMID: 40281704 Free PMC article.
-
Pulsta Valve, Unique Self-Expandable Transcatheter Pulmonary Valve.Korean Circ J. 2025 Aug;55(8):659-671. doi: 10.4070/kcj.2025.0083. Epub 2025 Apr 22. Korean Circ J. 2025. PMID: 40537432 Free PMC article. Review.
References
-
- Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. Task force on the management of grown-up congenital heart disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. (2010) 31(23):2915–57. 10.1093/eurheartj/ehq249 - DOI - PubMed
LinkOut - more resources
Full Text Sources

