Improved clinical trial race/ethnicity reporting and updated inclusion profile, 2017-2022: A New Jersey snapshot
- PMID: 38259323
- PMCID: PMC10801241
- DOI: 10.1016/j.gloepi.2023.100134
Improved clinical trial race/ethnicity reporting and updated inclusion profile, 2017-2022: A New Jersey snapshot
Abstract
Background: Diverse representation in clinical trials is an important goal in the testing of a medical, diagnostic, or therapeutic intervention. To date, the desired level of trial equity and inclusivity has been unevenly achieved.
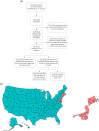
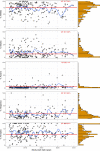
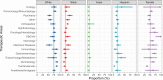
Methods: Employing the US National Library of Medicine's Clinicaltrials.gov registry, we examined 481 clinical trials conducted - at least in part - in the state of New Jersey. These trials were initiated after the FDA-mandated Common Rule changes, i.e., between January 2017 and October 2022, were enacted, and had their results posted. We analyzed sex/race/ethnicity reporting as well as applicable enrollment. Using meta-analysis, we estimated group participation proportions of a subset of the 481 identified trials; specifically, the 229 studies that were conducted solely within the US (i.e., without international sites) and compared them to US census data.
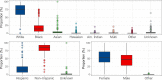
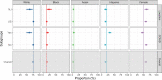
Findings: Within the 481 clinical trials analyzed, over 97% reported on the race and/or ethnicity of their enrollees; all included information on sex. Reporting was not affected by funding source or therapeutic area. Based on the 229 solely US-based studies, the participants overall were 76.7% White; 14.1% Black; 2.7% Asian; and 15% Hispanic. Inclusion of Black participants did not differ from the 2020 US census data; in contrast, the levels of Asian and Hispanic participation were below the corresponding census percentages.
Interpretation: The past five years have seen an overall uptick in the equity of race/ethnicity reporting and inclusivity of clinical trials, as compared to previously reported data, presaging the potential acquisition of ever more powerful and meaningful results of such interventional studies going forward.
Funding: Support for this study comes from the Hackensack Meridian Health Research Institute and the Hackensack Meridian School of Medicine.
Research in context: Evidence before this studyClinical trials are a critical part of determining whether or not a medical (drug/device/biologic) or socio-behavioral intervention is safe and truly effective. Through their use, scientific understanding is advanced and, ideally, human health is improved. To gain the most impactful information from a clinical trial, it should be sufficiently representative, that is, should enroll an adequate number of participants, and include a diverse population. Without such inclusion, the study is of only limited generalizability. Efforts are underway by funders, sites, and other stakeholders, to enhance reporting and promote inclusive enrollment. The extent to which such attempts are yielding results - at least for clinical trials in the state of New Jersey - is the focus of this data-driven analysis. The ClinicalTrials.gov registry database was carefully mined for the information contained in this report.Added value of this studyOur analysis of clinical trials initiated in the state of New Jersey and conducted there or elsewhere in the US reveals several positive trends. Our 5-year snapshot reveals that a very large percentage of trials report on race/ethnicity - and inclusivity is improving. While there is still some way to go to have the demographic numbers in these trials match US census values, our results suggest that recent efforts are having an effect.Implications of all the available evidenceFor myriad reasons, clinical trials have not enjoyed the public's universal trust over the years. In many ways, medicine moves at the speed of trust - without it, the promise of modern healthcare is brought into question. Clinical trials must include a commitment to diverse enrollment pools and equitable reporting under the law. Creating a legacy of trust - through greater inclusivity in clinical trials and more transparent reporting of results - will begin to heal the divide and engender faith in modern medicine and today's healthcare system. It would also allow for the desired far-reaching generalizability of results across patient populations. To better appreciate what needs to be done going forward, we must truly understand the state of clinical trials reporting and demographic inclusion. This report initiates such an analysis, by carefully documenting how New Jersey's clinical trials are performing. By virtue of its location (e.g., proximity to the cities of New York and Philadelphia) the state is part of a large biopharma cluster and healthcare nexus; it is critical that it performs well with respect to adopting/adhering to updated clinical trial guideline mandates. This report provides a glimpse - an important first look - into the state of clinical trials in New Jersey - from 2017 through 2022.
Keywords: Clinical trials; Clinicaltrials.gov; Ethnicity; Evidence-based medicine; Race.
© 2023 Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing/conflicting interests.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Is Our Science Representative? A Systematic Review of Racial and Ethnic Diversity in Orthopaedic Clinical Trials from 2000 to 2020.Clin Orthop Relat Res. 2022 May 1;480(5):848-858. doi: 10.1097/CORR.0000000000002050. Epub 2021 Dec 2. Clin Orthop Relat Res. 2022. PMID: 34855650 Free PMC article.
-
Demographic diversity of US-based participants in GSK-sponsored interventional clinical trials.Clin Trials. 2023 Apr;20(2):133-144. doi: 10.1177/17407745221149118. Epub 2023 Feb 6. Clin Trials. 2023. PMID: 36744680 Free PMC article.
-
Reporting of Participant Race and Ethnicity in Published US Pediatric Clinical Trials From 2011 to 2020.JAMA Pediatr. 2022 May 1;176(5):e220142. doi: 10.1001/jamapediatrics.2022.0142. Epub 2022 May 2. JAMA Pediatr. 2022. PMID: 35311946 Free PMC article.
-
Reporting results in U.S. clinical trials for obstructive sleep apnea and insomnia: How transparent are they?Sleep Health. 2020 Aug;6(4):529-533. doi: 10.1016/j.sleh.2019.11.009. Epub 2020 Mar 14. Sleep Health. 2020. PMID: 32179065 Free PMC article. Review.
Cited by
-
Health disparities and associated social determinants of health in interstitial lung disease: a narrative review.Eur Respir Rev. 2025 Apr 2;34(176):240176. doi: 10.1183/16000617.0176-2024. Print 2025 Apr. Eur Respir Rev. 2025. PMID: 40174956 Free PMC article. Review.
-
Diversity in Axial Spondyloarthritis Drug Trials: Enrollment by Sex, Race, Ethnicity, and Geographic Region.J Rheumatol. 2025 Mar 15:jrheum.2024-1013. doi: 10.3899/jrheum.2024-1013. Online ahead of print. J Rheumatol. 2025. PMID: 40089303
References
-
- U.S. Food and Drug Administration Food and Drug Administration Amendments Act (FDAAA) of 2007. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/f... [Accessed 2nd March 2023]
-
- Federal Register Clinical trials registration and results information submission. https://www.federalregister.gov/documents/2016/09/21/2016-22129/clinical... [Accessed 2nd March 2023] - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

