Polymeric nanocapsules loaded with poly(I:C) and resiquimod to reprogram tumor-associated macrophages for the treatment of solid tumors
- PMID: 38259462
- PMCID: PMC10800412
- DOI: 10.3389/fimmu.2023.1334800
Polymeric nanocapsules loaded with poly(I:C) and resiquimod to reprogram tumor-associated macrophages for the treatment of solid tumors
Abstract
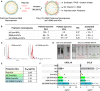
Background: In the tumor microenvironment (TME), tumor-associated macrophages (TAMs) play a key immunosuppressive role that limits the ability of the immune system to fight cancer. Toll-like receptors (TLRs) ligands, such as poly(I:C) or resiquimod (R848) are able to reprogram TAMs towards M1-like antitumor effector cells. The objective of our work has been to develop and evaluate polymeric nanocapsules (NCs) loaded with poly(I:C)+R848, to improve drug stability and systemic toxicity, and evaluate their targeting and therapeutic activity towards TAMs in the TME of solid tumors.
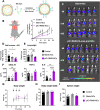
Methods: NCs were developed by the solvent displacement and layer-by-layer methodologies and characterized by dynamic light scattering and nanoparticle tracking analysis. Hyaluronic acid (HA) was chemically functionalized with mannose for the coating of the NCs to target TAMs. NCs loaded with TLR ligands were evaluated in vitro for toxicity and immunostimulatory activity by Alamar Blue, ELISA and flow cytometry, using primary human monocyte-derived macrophages. For in vivo experiments, the CMT167 lung cancer model and the MN/MCA1 fibrosarcoma model metastasizing to lungs were used; tumor-infiltrating leukocytes were evaluated by flow cytometry and multispectral immunophenotyping.
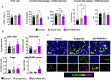
Results: We have developed polymeric NCs loaded with poly(I:C)+R848. Among a series of 5 lead prototypes, protamine-NCs were selected based on their physicochemical properties (size, charge, stability) and in vitro characterization, showing good biocompatibility on primary macrophages and ability to stimulate their production of T-cell attracting chemokines (CXCL10, CCL5) and to induce M1-like macrophages cytotoxicity towards tumor cells. In mouse tumor models, the intratumoral injection of poly(I:C)+R848-protamine-NCs significantly prevented tumor growth and lung metastasis. In an orthotopic murine lung cancer model, the intravenous administration of poly(I:C)+R848-prot-NCs, coated with an additional layer of HA-mannose to improve TAM-targeting, resulted in good antitumoral efficacy with no apparent systemic toxicity. While no significant alterations were observed in T cell numbers (CD8, CD4 or Treg), TAM-reprogramming in treated mice was confirmed by the relative decrease of interstitial versus alveolar macrophages, having higher CD86 expression but lower CD206 and Arg1 expression in the same cells, in treated mice.
Conclusion: Mannose-HA-protamine-NCs loaded with poly(I:C)+R848 successfully reprogram TAMs in vivo, and reduce tumor progression and metastasis spread in mouse tumors.
Keywords: antitumoral immunotherapy; cancer; immunotoxicology; poly(I:C); polymeric nanocapsules; resiquimod (R848); toll-like receptor (TLR); tumor-associated macrophages (TAMs).
Copyright © 2024 Anfray, Varela, Ummarino, Maeda, Sironi, Gandoy, Brea, Loza, León, Calvo, Correa, Fernandez-Megia, Alonso, Allavena, Crecente-Campo and Andón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

