Chronic almond nut snacking alleviates perceived muscle soreness following downhill running but does not improve indices of cardiometabolic health in mildly overweight, middle-aged, adults
- PMID: 38260074
- PMCID: PMC10800814
- DOI: 10.3389/fnut.2023.1298868
Chronic almond nut snacking alleviates perceived muscle soreness following downhill running but does not improve indices of cardiometabolic health in mildly overweight, middle-aged, adults
Abstract
Introduction: As a popular food snack rich in protein, fiber, unsaturated fatty acids, antioxidants and phytonutrients, almond nut consumption is widely associated with improvements in cardiometabolic health. However, limited data exists regarding the role of almond consumption in improving exercise recovery. Accordingly, we aimed to investigate the impact of chronic almond snacking on muscle damage and cardiometabolic health outcomes during acute eccentric exercise recovery in mildly overweight, middle-aged, adults.
Methods: Using a randomized cross-over design, 25 mildly overweight (BMI: 25.8 ± 3.6 kg/m2), middle-aged (35.1 ± 4.7 y) males (n = 11) and females (n = 14) performed a 30-min downhill treadmill run after 8-weeks of consuming either 57 g/day of whole almonds (ALMOND) or an isocaloric amount (86 g/day) of unsalted pretzels (CONTROL). Muscle soreness (visual analogue scale), muscle function (vertical jump and maximal isokinetic torque) and blood markers of muscle damage (creatine kinase (CK) concentration) and inflammation (c-reactive protein concentration) were measured pre and post (24, 48, and 72 h) exercise. Blood biomarkers of cardiometabolic health (total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol), body composition and psycho-social assessments of mood (POMS-2 inventory), appetite and well-being were measured pre and post intervention.
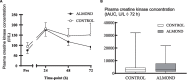
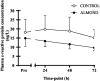
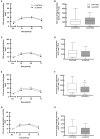
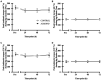
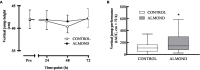
Results: Downhill running successfully elicited muscle damage, as evidenced by a significant increase in plasma CK concentration, increased perception of muscle soreness, and impaired vertical jump performance (all p < 0.05) during acute recovery. No effect of trial order was observed for any outcome measurement. However, expressed as AUC over the cumulative 72 h recovery period, muscle soreness measured during a physical task (vertical jump) was reduced by ~24% in ALMOND vs. CONTROL (p < 0.05) and translated to an improved maintenance of vertical jump performance (p < 0.05). However, ALMOND did not ameliorate the CK response to exercise or isokinetic torque during leg extension and leg flexion (p > 0.05). No pre-post intervention changes in assessments of cardiometabolic health, body composition, mood state or appetite were observed in ALMOND or CONTROL (all p > 0.05).
Conclusion: Chronic almond supplementation alleviates task-specific perceived feelings of muscle soreness during acute recovery from muscle damaging exercise, resulting in the better maintenance of muscle functional capacity. These data suggest that almonds represent a functional food snack to improve exercise tolerance in mildly overweight, middle-aged adults.
Keywords: appetite; body composition; exercise tolerance; functional foods; muscle damage.
Copyright © 2024 Siegel, Rooney, Marjoram, Mason, Bowles, van Keulen, Helander, Rayo, Hong, Liu, Hooshmand, Kern and Witard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Almond Consumption Modestly Improves Pain Ratings, Muscle Force Production, and Biochemical Markers of Muscle Damage Following Downhill Running in Mildly Overweight, Middle-Aged Adults: A Randomized, Crossover Trial.Curr Dev Nutr. 2024 Aug 7;8(9):104432. doi: 10.1016/j.cdnut.2024.104432. eCollection 2024 Sep. Curr Dev Nutr. 2024. PMID: 39257478 Free PMC article.
-
Pistachios as a recovery food following downhill running exercise in recreational team-sport individuals.Eur J Sport Sci. 2023 Dec;23(12):2400-2410. doi: 10.1080/17461391.2023.2239192. Epub 2023 Aug 18. Eur J Sport Sci. 2023. PMID: 37596062 Clinical Trial.
-
Fish oil supplementation fails to modulate indices of muscle damage and muscle repair during acute recovery from eccentric exercise in trained young males.Eur J Sport Sci. 2023 Aug;23(8):1666-1676. doi: 10.1080/17461391.2023.2199282. Epub 2023 Apr 25. Eur J Sport Sci. 2023. PMID: 37010103
-
Muscle tenderness and peak torque changes after downhill running following a prior bout of isokinetic eccentric exercise.J Sports Sci. 1996 Aug;14(4):291-9. doi: 10.1080/02640419608727714. J Sports Sci. 1996. PMID: 8887208 Clinical Trial.
-
The Effects of Pre-conditioning on Exercise-Induced Muscle Damage: A Systematic Review and Meta-analysis.Sports Med. 2023 Aug;53(8):1537-1557. doi: 10.1007/s40279-023-01839-8. Epub 2023 May 9. Sports Med. 2023. PMID: 37160563 Free PMC article.
Cited by
-
Effects of Almond Consumption on Selected Markers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis.Clin Nutr Res. 2025 Jan 31;14(1):78-89. doi: 10.7762/cnr.2025.14.1.78. eCollection 2025 Jan. Clin Nutr Res. 2025. PMID: 39968278 Free PMC article. Review.
-
Polyphenol-Rich Snack Consumption during Endurance Exercise Training Improves Nitric Oxide Bioavailability but does not Improve Exercise Performance in Male Cyclists: A Randomised Controlled Trial.Curr Dev Nutr. 2025 Mar 24;9(5):106006. doi: 10.1016/j.cdnut.2025.106006. eCollection 2025 May. Curr Dev Nutr. 2025. PMID: 40321836 Free PMC article.
-
Almond Consumption Modestly Improves Pain Ratings, Muscle Force Production, and Biochemical Markers of Muscle Damage Following Downhill Running in Mildly Overweight, Middle-Aged Adults: A Randomized, Crossover Trial.Curr Dev Nutr. 2024 Aug 7;8(9):104432. doi: 10.1016/j.cdnut.2024.104432. eCollection 2024 Sep. Curr Dev Nutr. 2024. PMID: 39257478 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

