Tomato-made edible COVID-19 vaccine TOMAVAC induces neutralizing IgGs in the blood sera of mice and humans
- PMID: 38260078
- PMCID: PMC10800535
- DOI: 10.3389/fnut.2023.1275307
Tomato-made edible COVID-19 vaccine TOMAVAC induces neutralizing IgGs in the blood sera of mice and humans
Abstract
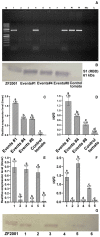
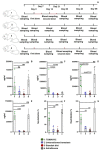
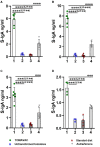
Plant-based edible vaccines that provide two-layered protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outweigh the currently used parenteral types of vaccines, which predominantly cause a systemic immune response. Here, we engineered and selected a transgenic tomato genotype (TOMAVAC) that stably synthesized an antigenic S1 protein of SARS-CoV-2. Two-course spaced force-feeding of mice with ≈5.4 μg/ml TOMAVAC increased up to 16-fold the synthesis of RBD-specific NAbs in blood serum and the significant induction of S-IgA in intestinal lavage fluid. In a surrogate virus neutralization test, TOMAVAC-induced NAbs had 15-25% viral neutralizing activity. The results suggested early evidence of the immunogenicity and protectivity of TOMAVAC against the coronavirus disease 2019 (COVID-19) infection. Furthermore, we observed a positive trend of statistically significant 1.2-fold (average of +42.28 BAU/ml) weekly increase in NAbs in the volunteers' serum relative to the initial day. No severe side effects were observed, preliminarily supporting the safety of TOMAVAC. With the completion of future large-scale studies, higher-generation TOMAVAC should be a cost-effective, ecologically friendly, and widely applicable novel-generation COVID-19 vaccine, providing two-layered protection against SARS-CoV-2.
Keywords: Edible vaccine; RBD-specific neutralizing antibodies; SARS-CoV-2; efficiency; safety; viral neutralizing activity.
Copyright © 2024 Buriev, Shermatov, Usmanov, Mirzakhmedov, Ubaydullaeva, Kamburova, Rakhmanov, Ayubov, Abdullaev, Eshmurzaev, Mamajonov, Tulanov, Ismailova, Petrova, Rozumbetov, Aripova, Muminov, Ermatova, Dalimova, Turdikulova, Abdukarimov and Abdurakhmonov.
Conflict of interest statement
The study concept and results have been filed for patenting at the Intellectual Property Agency under the Ministry of Justice of the Republic of Uzbekistan with pending application no. IAP 2022 0247.
Figures

Similar articles
-
Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients.BMC Med. 2021 Aug 23;19(1):208. doi: 10.1186/s12916-021-02090-6. BMC Med. 2021. PMID: 34420521 Free PMC article.
-
A mosaic-type trimeric RBD-based COVID-19 vaccine candidate induces potent neutralization against Omicron and other SARS-CoV-2 variants.Elife. 2022 Aug 25;11:e78633. doi: 10.7554/eLife.78633. Elife. 2022. PMID: 36004719 Free PMC article.
-
Elicitation of Broadly Neutralizing Antibodies against B.1.1.7, B.1.351, and B.1.617.1 SARS-CoV-2 Variants by Three Prototype Strain-Derived Recombinant Protein Vaccines.Viruses. 2021 Jul 22;13(8):1421. doi: 10.3390/v13081421. Viruses. 2021. PMID: 34452287 Free PMC article.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
Most accurate mutations in SARS-CoV-2 genomes identified in Uzbek patients show novel amino acid changes.Front Med (Lausanne). 2024 May 31;11:1401655. doi: 10.3389/fmed.2024.1401655. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38882660 Free PMC article.
-
Development of SARS-CoV-2 neutralizing antibody detection assay by using recombinant plant-produced proteins.Biotechnol Rep (Amst). 2023 Jun;38:e00796. doi: 10.1016/j.btre.2023.e00796. Epub 2023 Apr 6. Biotechnol Rep (Amst). 2023. PMID: 37056791 Free PMC article.
-
Simple and Fail-safe Method to Transform Miniprep Escherichia coli Strain K12 Plasmid DNA Into Viable Agrobacterium tumefaciens EHA105 Cells for Plant Genetic Transformation.Bio Protoc. 2025 Jan 5;15(1):e5174. doi: 10.21769/BioProtoc.5174. eCollection 2025 Jan 5. Bio Protoc. 2025. PMID: 39803315 Free PMC article.
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). (2022) Available online at: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (accessed December 22, 2022).
LinkOut - more resources
Full Text Sources
Miscellaneous

