Evaluation of adipogenesis over time using a novel bioabsorbable implant without the addition of exogenous cells or growth factors
- PMID: 38260087
- PMCID: PMC10801210
- DOI: 10.1016/j.reth.2023.12.015
Evaluation of adipogenesis over time using a novel bioabsorbable implant without the addition of exogenous cells or growth factors
Abstract
Background: Breast reconstruction is crucial for patients who have undergone mastectomy for breast cancer. Our bioabsorbable implants comprising an outer poly-l-lactic acid mesh and an inner component filled with collagen sponge promote and retain adipogenesis in vivo without the addition of exogenous cells or growth factors. In this study, we evaluated adipogenesis over time histologically and at the gene expression level using this implant in a rodent model.
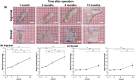
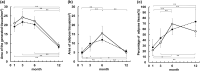
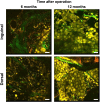
Methods: The implants were inserted in the inguinal and dorsal regions of the animals. At 1, 3, 6, and 12 months post-operation, the weight, volume, and histological assessment of all newly formed tissue were performed. We analyzed the formation of new adipose tissue using multiphoton microscopy and RNA sequencing.
Results: Both in the inguinal and dorsal regions, adipose tissue began to form 1 month post-operation in the peripheral area. Angiogenesis into implants was observed until 3 months. At 6 months, microvessels matured and the amount of newly generated adipose tissue peaked and was uniformly distributed inside implants. The amount of newly generated adipose tissue decreased from 6 to 12 months but at 12 months, adipose tissue was equivalent to the native tissue histologically and in terms of gene expression.
Conclusions: Our bioabsorbable implants could induce normal adipogenesis into the implants after subcutaneous implantation. Our implants can serve as a novel and safe material for breast reconstruction without requiring exogenous cells or growth factors.
Keywords: Adipogenesis; Bioabsorbable; Breast cancer; Multiphoton excitation fluorescence microscopy.
© 2023 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Figures






Similar articles
-
Adipogenesis of bioabsorbable implants under irradiation in a rodent model.Regen Ther. 2024 Oct 30;26:990-998. doi: 10.1016/j.reth.2024.10.002. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39524181 Free PMC article.
-
Preliminary report of de novo adipogenesis using novel bioabsorbable implants and image evaluation using a porcine model.J Artif Organs. 2022 Sep;25(3):245-253. doi: 10.1007/s10047-022-01313-8. Epub 2022 Mar 2. J Artif Organs. 2022. PMID: 35235081 Free PMC article.
-
Long term observation of de novo adipogenesis using novel bioabsorbable implants with larger size in a porcine model.Regen Ther. 2023 Aug 24;24:324-331. doi: 10.1016/j.reth.2023.08.004. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37649673 Free PMC article.
-
De novo adipogenesis using a bioabsorbable implant without additional cells or growth factors.J Tissue Eng Regen Med. 2020 Jul;14(7):920-930. doi: 10.1002/term.3041. Epub 2020 Jun 3. J Tissue Eng Regen Med. 2020. PMID: 32293793
-
Development of a novel bioabsorbable implant that is substituted by adipose tissue in vivo.J Tissue Eng Regen Med. 2018 Mar;12(3):633-641. doi: 10.1002/term.2482. Epub 2017 Sep 28. J Tissue Eng Regen Med. 2018. PMID: 28548695
Cited by
-
Adipogenesis of bioabsorbable implants under irradiation in a rodent model.Regen Ther. 2024 Oct 30;26:990-998. doi: 10.1016/j.reth.2024.10.002. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39524181 Free PMC article.
References
-
- Silverman B.G., Brown S.L., Bright R.A., Kaczmarek R.G., Arrowsmith-Lowe J.B., Kessler D.A. Reported complications of silicone gel breast implants: an epidemiologic review. Ann Intern Med. 1996;124:744–756. - PubMed
-
- Brown S.L., Silverman B.G., Berg W.A. Rupture of silicone-gel breast implants: causes, sequelae, and diagnosis. Lancet. 1997;350:1531–1537. - PubMed
-
- Alotaibi S., Hamadani M., Al-Mansour M., Aljurf M. Breast implant-associated anaplastic large cell lymphoma. Clin Lymphoma, Myeloma Leukemia. 2021;21:e272–e276. - PubMed
-
- Ellenbogen R. Free autogenous pearl fat grafts in the face--a preliminary report of a rediscovered technique. Ann Plast Surg. 1986;16:179–194. - PubMed
LinkOut - more resources
Full Text Sources

