Investigating G-protein coupled receptor signalling with light-emitting biosensors
- PMID: 38260094
- PMCID: PMC10801095
- DOI: 10.3389/fphys.2023.1310197
Investigating G-protein coupled receptor signalling with light-emitting biosensors
Abstract
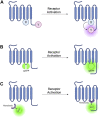
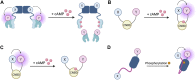
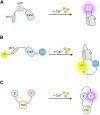
G protein-coupled receptors (GPCRs) are the most frequent target of currently approved drugs and play a central role in both physiological and pathophysiological processes. Beyond the canonical understanding of GPCR signal transduction, the importance of receptor conformation, beta-arrestin (β-arr) biased signalling, and signalling from intracellular locations other than the plasma membrane is becoming more apparent, along with the tight spatiotemporal compartmentalisation of downstream signals. Fluorescent and bioluminescent biosensors have played a pivotal role in elucidating GPCR signalling events in live cells. To understand the mechanisms of action of the GPCR-targeted drugs currently available, and to develop new and better GPCR-targeted therapeutics, understanding these novel aspects of GPCR signalling is critical. In this review, we present some of the tools available to interrogate each of these features of GPCR signalling, we illustrate some of the key findings which have been made possible by these tools and we discuss their limitations and possible developments.
Keywords: BRET; FRET; G protein; GPCR; bioluminescence; fluorescent biosensor; signalling.
Copyright © 2024 Demby and Zaccolo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Genetically encoded fluorescent biosensors for GPCR research.Front Cell Dev Biol. 2022 Sep 29;10:1007893. doi: 10.3389/fcell.2022.1007893. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36247000 Free PMC article. Review.
-
Quantitative approaches for studying G protein-coupled receptor signalling and pharmacology.J Cell Sci. 2025 Jan 1;138(1):JCS263434. doi: 10.1242/jcs.263434. Epub 2025 Jan 15. J Cell Sci. 2025. PMID: 39810711 Free PMC article. Review.
-
Detecting GPCR Signals With Optical Biosensors of Gα-GTP in Cell Lines and Primary Cell Cultures.Curr Protoc. 2023 Jun;3(6):e796. doi: 10.1002/cpz1.796. Curr Protoc. 2023. PMID: 37310083 Free PMC article.
-
BRET biosensors to study GPCR biology, pharmacology, and signal transduction.Front Endocrinol (Lausanne). 2012 Aug 29;3:105. doi: 10.3389/fendo.2012.00105. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22952466 Free PMC article.
-
Spatiotemporal GPCR signaling illuminated by genetically encoded fluorescent biosensors.Curr Opin Pharmacol. 2023 Aug;71:102384. doi: 10.1016/j.coph.2023.102384. Epub 2023 Jun 14. Curr Opin Pharmacol. 2023. PMID: 37327640 Review.
Cited by
-
Ligand-Induced Biased Activation of GPCRs: Recent Advances and New Directions from In Silico Approaches.Molecules. 2025 Feb 25;30(5):1047. doi: 10.3390/molecules30051047. Molecules. 2025. PMID: 40076272 Free PMC article. Review.
-
Nanodomain cAMP signaling in cardiac pathophysiology: potential for developing targeted therapeutic interventions.Physiol Rev. 2025 Apr 1;105(2):541-591. doi: 10.1152/physrev.00013.2024. Epub 2024 Aug 8. Physiol Rev. 2025. PMID: 39115424 Free PMC article. Review.
-
Direct effect of membrane environment on the activation of mGluR2 revealed by single-molecule FRET.Structure. 2025 Apr 3;33(4):718-727.e4. doi: 10.1016/j.str.2025.01.011. Epub 2025 Feb 4. Structure. 2025. PMID: 39909029
-
GPCR signaling via cAMP nanodomains.Biochem J. 2025 May 13;482(10):519-33. doi: 10.1042/BCJ20253088. Biochem J. 2025. PMID: 40364615 Free PMC article. Review.
References
-
- Angers S., Salahpour A., Joly E., Hilairet S., Chelsky D., Dennis M., et al. (2000). Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc. Natl. Acad. Sci. U. S. A. 97, 3684–3689. 10.1073/pnas.060590697 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

