Enlarged colony housing promotes linear progression of subchondral bone remodeling in joint instability rat models
- PMID: 38260097
- PMCID: PMC10800552
- DOI: 10.3389/fphys.2023.1232416
Enlarged colony housing promotes linear progression of subchondral bone remodeling in joint instability rat models
Abstract
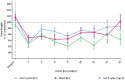
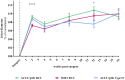
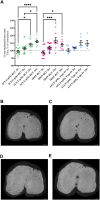
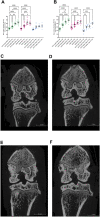
Objective: Osteoarthritis (OA) is a disease with high prevalence and an unmet medical need for disease modifying treatments. In rat models, OA-like subchondral bone and cartilage changes can be induced by instability surgery with different severity levels. Factors which determine structural changes additionally comprise the study duration and activity-impacted joint loading. Methods: A medial meniscal tear (MMT) or anterior cruciate ligament transection with partial meniscectomy (ACLT+pMx) was induced unilaterally in rats housed in a rat colony cage (RCC), allowing high activity levels including jumping and stair climbing. In parallel, ACLT+pMx rats were housed in Type IV cages. The time course of OA-related changes was investigated at 4, 8, 12, and 16 weeks after surgery by micro-CT, gait analysis and joint diameter measurements. Results: Gait disturbance was observed after 2 weeks and to a similar extend in all models. The increase in ipsilateral joint diameters peaked after 2 weeks and were more pronounced after ACLT+pMx compared to MMT-surgery, but independent of housing. Micro-CT analysis revealed that increases in osseous tibial width were most distinct after ACLT+pMx in RCC and progressed continuously until week sixteen. In contrast, osseous tibial width of ipsilateral joints in MMT RCC and ACLT+pMx Type IV groups did not increase further after week twelve. In contralateral joints, this parameter was not affected, regardless of the model or caging. However, a significant increase in bone volume fraction and trabecular thickness was observed over time in the femur and tibia of both ipsilateral and contralateral knees. Here, the medial tibial compartment of the operated joint was most affected and linear changes were most pronounced in the ACLT+pMx RCC group. Conclusion: Increased movement of animals in colony cages leads to robust structural changes in subchondral bone after surgically induced joint instability over time, while in regular Type IV housing maximal changes are reached in week twelve. The new insights into the differentiation of the models, particularly with respect to the linear progression of bone changes in ACLT+pMx in the RCC, may be useful for the design of chronic OA-studies within a longer lifespan and therefore supporting the development of novel therapies.
Keywords: colony housing; joint instability; osteoarthritis; rats; subchondral bone.
Copyright © 2024 Menges, Kleinschmidt-Dörr and Brenneis.
Conflict of interest statement
The authors SM and CB are employees of Merck Healthcare KGaA. KK-D is employed by Merck KGaA. This work was sponsored by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef funder ID: 10.13039/100009945). Representatives of the sponsor were involved in the study design, collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Figures
Similar articles
-
Colony housing promotes structural and functional changes during surgically induced osteoarthritis in rats.Osteoarthr Cartil Open. 2020 Sep 16;2(4):100100. doi: 10.1016/j.ocarto.2020.100100. eCollection 2020 Dec. Osteoarthr Cartil Open. 2020. PMID: 36474887 Free PMC article.
-
Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis.Bone. 2006 Feb;38(2):234-43. doi: 10.1016/j.bone.2005.08.007. Epub 2005 Sep 26. Bone. 2006. PMID: 16185945
-
Effects of calcitonin on subchondral trabecular bone changes and on osteoarthritic cartilage lesions after acute anterior cruciate ligament deficiency.J Bone Miner Res. 2004 Nov;19(11):1821-6. doi: 10.1359/JBMR.040609. Epub 2004 Jun 28. J Bone Miner Res. 2004. PMID: 15476582
-
Systematic analysis reveals that colony housing aligns gait profiles and strengthens link between histological and micro-CT bone markers in rat models of osteoarthritis.FASEB J. 2021 Apr;35(4):e21451. doi: 10.1096/fj.202002009R. FASEB J. 2021. PMID: 33683776
-
Molecular changes in articular cartilage and subchondral bone in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis.BMC Musculoskelet Disord. 2011 Aug 24;12:197. doi: 10.1186/1471-2474-12-197. BMC Musculoskelet Disord. 2011. PMID: 21864409 Free PMC article.
References
-
- Bendele A. M. (2001). Animal models of osteoarthritis. J. Musculoskel Neuron Interact. 1, 363–376. - PubMed
LinkOut - more resources
Full Text Sources

