Intramuscular injection of human chorionic gonadotropin as luteal phase support in artificial cycle frozen-thawed embryo transfer does not improve clinical outcomes: a parallel, open-label randomized trial
- PMID: 38260168
- PMCID: PMC10801214
- DOI: 10.3389/fendo.2023.1283197
Intramuscular injection of human chorionic gonadotropin as luteal phase support in artificial cycle frozen-thawed embryo transfer does not improve clinical outcomes: a parallel, open-label randomized trial
Abstract
Background: Human chorionic gonadotropin (hCG) as one of the first signals secreted by the embryo to the mother may have a direct effect on the endometrium at implantation. The current study was aim to compare the clinical outcomes after frozen-thawed embryo transfer (FET) treated with artificial cycles (AC) between women who were administered intramuscular injection of human chorionic gonadotropin (hCG) as luteal phase support and the routine group.
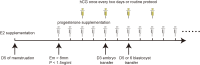
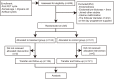
Methods: A randomized controlled trial of 245 women was conducted at the Assisted Reproduction Center, Northwest Women's and Children's Hospital, Xi'an, China from January 2019 to January 2020. Women <40 years of age undergoing their first FET treated with AC were included. Patients were randomly allocated into either: (1) the hCG treatment group, who received intramuscular injection of hCG since the third day of progesterone administration, at a dose of 2000 IU once every two days, for a total of four times, (2) the control group, receiving routine protocol without placebo on these four days. Clinical outcomes of the two groups were analyzed.
Results: The primary outcome ongoing pregnancy rate in the hCG treatment group versus the control group was 73/124 (58.87%) versus 75/121 (61.98%), respectively (odds ratio [OR], 95% confidence interval [CI]:0.88, 0.53-1.47, P = 0.619). Secondary clinical outcomes including biochemical pregnancy, clinical pregnancy, early pregnancy loss, multiple pregnancy, live birth and preterm birth were also comparable between the two groups through the univariate analysis and multivariable regression analysis (P > 0.05).
Conclusion: In women undergoing AC-FET, there was no significant difference in the clinical outcomes between the hCG treatment group and the control group. Clinicians should be cautious about adding IM-hCG as luteal phase support to improve the clinical outcome after AC-FET.
Clinical trial registration: http://www.chictr.org.cn/showprojen.aspx?proj=32511, identifier ChiCTR1800020342.
Keywords: artificial cycle; frozen embryo transfer; intramuscular injection of hCG; ongoing pregnancy rate; randomized controlled trial.
Copyright © 2024 Li, Huang, Shi, Shi and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Salemi S, Yahyaei A, Vesali S, Ghaffari F. Endometrial preparation for vitrified-warmed embryo transfer with or without gnrh-agonist pre-treatment in patients with polycystic ovary syndrome: A randomized controlled trial. Reprod BioMed Online (2021) 43(3):446–52. doi: 10.1016/j.rbmo.2021.06.006 - DOI - PubMed
-
- Azimi Nekoo E, Chamani M, Shahrokh Tehrani E, Hossein Rashidi B, Davari Tanha F, Kalantari V. Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Family Reprod Health (2015) 9(1):1–4. - PMC - PubMed
-
- Nakajima ST, Nason FG, Badger GJ, Gibson M. Progesterone production in early pregnancy. Fertil Steril (1991) 55(3):516–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources