Characterization of three predicted zinc exporters in Brucella ovis identifies ZntR-ZntA as a powerful zinc and cadmium efflux system not required for virulence and unveils pathogenic Brucellae heterogeneity in zinc homeostasis
- PMID: 38260206
- PMCID: PMC10800456
- DOI: 10.3389/fvets.2023.1323500
Characterization of three predicted zinc exporters in Brucella ovis identifies ZntR-ZntA as a powerful zinc and cadmium efflux system not required for virulence and unveils pathogenic Brucellae heterogeneity in zinc homeostasis
Abstract
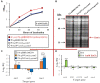
Brucella ovis causes non-zoonotic ovine brucellosis of worldwide distribution and is responsible for important economic losses mainly derived from male genital lesions and reproductive fails. Studies about the virulence mechanisms of this rough species (lacking lipopolysaccharide O-chains) are underrepresented when compared to the main zoonotic Brucella species that are smooth (with O-chains). Zinc intoxication constitutes a defense mechanism of the host against bacterial pathogens, which have developed efflux systems to counterbalance toxicity. In this study, we have characterized three potential B. ovis zinc exporters, including the ZntA ortholog previously studied in B. abortus. Despite an in-frame deletion removing 100 amino acids from B. ovis ZntA, the protein retained strong zinc efflux properties. Only indirect evidence suggested a higher exporter activity for B. abortus ZntA, which, together with differences in ZntR-mediated regulation of zntA expression between B. ovis and B. abortus, could contribute to explaining why the ΔzntR mutant of B. abortus is attenuated while that of B. ovis is virulent. Additionally, B. ovis ZntA was revealed as a powerful cadmium exporter contributing to cobalt, copper, and nickel detoxification, properties not previously described for the B. abortus ortholog. Deletion mutants for BOV_0501 and BOV_A1100, also identified as potential zinc exporters and pseudogenes in B. abortus, behaved as the B. ovis parental strain in all tests performed. However, their overexpression in the ΔzntA mutant allowed the detection of discrete zinc and cobalt efflux activity for BOV_0501 and BOV_A1100, respectively. Nevertheless, considering their low expression levels and the stronger activity of ZntA as a zinc and cobalt exporter, the biological role of BOV_0501 and BOV_A1100 is questionable. Results presented in this study evidence heterogeneity among pathogenic Brucellae regarding zinc export and, considering the virulence of B. ovis ΔzntA, suggest that host-mediated zinc intoxication is not a relevant mechanism to control B. ovis infection.
Keywords: Brucella ovis; ZntA; ZntR; ZnuA; cadmium; cobalt; virulence; zinc.
Copyright © 2024 Tartilán-Choya, Tejedor, Conde-Álvarez, Muñoz and Vizcaíno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- OIE . Chapter 3.8.7. Ovine epididymitis (Brucella ovis). Manual of diagnostic tests and vaccines for terrestrial animals. (2021). Available at: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.7_OVIN....
-
- OIE . Chapter 3.1.4. Brucellosis (infection with B. abortus, B. Melitensis and B. suis). Manual of diagnostic tests and vaccines for terrestrial animals. (2021). Available at: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.4_BRUC....
LinkOut - more resources
Full Text Sources

