This is a preprint.
Skeletal muscle reprogramming enhances reinnervation after peripheral nerve injury
- PMID: 38260278
- PMCID: PMC10802751
- DOI: 10.21203/rs.3.rs-3463557/v1
Skeletal muscle reprogramming enhances reinnervation after peripheral nerve injury
Update in
-
Skeletal muscle reprogramming enhances reinnervation after peripheral nerve injury.Nat Commun. 2024 Oct 25;15(1):9218. doi: 10.1038/s41467-024-53276-4. Nat Commun. 2024. PMID: 39455585 Free PMC article.
Abstract
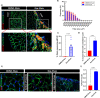
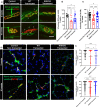
Peripheral Nerve Injuries (PNI) affect more than 20 million Americans and severely impact quality of life by causing long-term disability. The onset of PNI is characterized by nerve degeneration distal to the nerve injury resulting in long periods of skeletal muscle denervation. During this period, muscle fibers atrophy and frequently become incapable of "accepting" innervation because of the slow speed of axon regeneration post injury. We hypothesize that reprogramming the skeletal muscle to an embryonic-like state may preserve its reinnervation capability following PNI. To this end, we generated a mouse model in which NANOG, a pluripotency-associated transcription factor can be expressed locally upon delivery of doxycycline (Dox) in a polymeric vehicle. NANOG expression in the muscle upregulated the percentage of Pax7+ nuclei and expression of eMYHC along with other genes that are involved in muscle development. In a sciatic nerve transection model, NANOG expression led to upregulation of key genes associated with myogenesis, neurogenesis and neuromuscular junction (NMJ) formation, and downregulation of key muscle atrophy genes. Further, NANOG mice demonstrated extensive overlap between synaptic vesicles and NMJ acetylcholine receptors (AChRs) indicating restored innervation. Indeed, NANOG mice showed greater improvement in motor function as compared to wild-type (WT) animals, as evidenced by improved toe-spread reflex, EMG responses and isometric force production. In conclusion, we demonstrate that reprogramming the muscle can be an effective strategy to improve reinnervation and functional outcomes after PNI.
Figures




References
-
- Taylor C.A., et al., The incidence of peripheral nerve injury in extremity trauma. American journal of physical medicine & rehabilitation, 2008. 87(5): p. 381–385. - PubMed
-
- Rivera J.C., Glebus G., and Cho M., Disability following combat-sustained nerve injury of the upper limb. The bone & joint journal, 2014. 96(2): p. 254–258. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

