This is a preprint.
Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants
- PMID: 38260304
- PMCID: PMC10802657
- DOI: 10.1101/2024.01.08.24301002
Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants
Update in
-
Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants.Immunity. 2024 Apr 9;57(4):912-925.e4. doi: 10.1016/j.immuni.2024.02.017. Epub 2024 Mar 14. Immunity. 2024. PMID: 38490198
Abstract
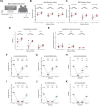
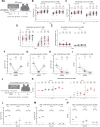
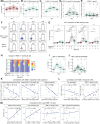
The spike glycoprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues to accumulate substitutions, leading to breakthrough infections of vaccinated individuals and prompting the development of updated booster vaccines. Here, we determined the specificity and functionality of antibody and B cell responses following exposure to BA.5 and XBB variants in individuals who received ancestral SARS-CoV-2 mRNA vaccines. BA.5 exposures elicited antibody responses that primarily targeted epitopes conserved between the BA.5 and ancestral spike, with poor reactivity to the XBB.1.5 variant. XBB exposures also elicited antibody responses that targeted epitopes conserved between the XBB.1.5 and ancestral spike. However, unlike BA.5, a single XBB exposure elicited low levels of XBB.1.5-specific antibodies and B cells in some individuals. Pre-existing cross-reactive B cells and antibodies were correlated with stronger overall responses to XBB but weaker XBB-specific responses, suggesting that baseline immunity influences the activation of variant-specific SARS-CoV-2 responses.
Keywords: B cells; Omicron BA.5; SARS-CoV-2; XBB.1.5; antibodies; immune evasion; immune imprinting; mRNA vaccines; original antigenic sin; viral evolution.
Conflict of interest statement
Declaration of Interests E.J.W. is a member of the Parker Institute for Cancer Immunotherapy. E.J.W. is an advisor for Arsenal Biosciences, Coherus, Danger Bio, IpiNovyx, Janssen, New Limit, Marengo, Pluto Immunotherapeutics Related Sciences, Santa Ana Bio, and Synthekine. E.J.W. is a founder of and holds stock in Coherus, Danger Bio, and Arsenal Biosciences.
Figures



Similar articles
-
Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants.Immunity. 2024 Apr 9;57(4):912-925.e4. doi: 10.1016/j.immuni.2024.02.017. Epub 2024 Mar 14. Immunity. 2024. PMID: 38490198
-
Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity.Cell Rep. 2023 Aug 29;42(8):112888. doi: 10.1016/j.celrep.2023.112888. Epub 2023 Jul 31. Cell Rep. 2023. PMID: 37527039
-
Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans.Immunity. 2024 Apr 9;57(4):904-911.e4. doi: 10.1016/j.immuni.2024.02.016. Epub 2024 Mar 14. Immunity. 2024. PMID: 38490197
-
Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron.Cytokine Growth Factor Rev. 2023 Apr;70:13-25. doi: 10.1016/j.cytogfr.2023.03.001. Epub 2023 Mar 5. Cytokine Growth Factor Rev. 2023. PMID: 36948931 Free PMC article. Review.
-
The "original antigenic sin" and its relevance for SARS-CoV-2 (COVID-19) vaccination.Clin Immunol Commun. 2021 Dec;1:13-16. doi: 10.1016/j.clicom.2021.10.001. Epub 2021 Oct 8. Clin Immunol Commun. 2021. PMID: 38620690 Free PMC article. Review.
References
-
- Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R. A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., Dinnon K.H., Elbashir S. M., Shaw C.A., Woods A., Fritch E.J., Martinez D.R., Bock K.W., Minai M., Nagata B.M., Hutchinson G.B., Wu K., Henry C., Bahl K., Garcia-Dominguez D., Ma L., Renzi I., Kong W.-P., Schmidt S.D., Wang L., Zhang Y., Phung E., Chang L.A., Loomis R.J., Altaras N.E., Narayanan E., Metkar M., Presnyak V., Liu C., Louder M. K., Shi W., Leung K., Yang E.S., West A., Gully K.L., Stevens L.J., Wang N., Wrapp D., Doria-Rose N.A., Stewart-Jones G., Bennett H., Alvarado G.S., Nason M.C., Ruckwardt T.J., McLellan J.S., Denison M.R., Chappell J.D., Moore I.N., Morabito K.M., Mascola J.R., Baric R.S., Carfi A., Graham B.S., 2020. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571. 10.1038/s41586-020-2622-0 - DOI - PMC - PubMed
-
- Hsieh C.-L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.-C., Javanmardi K., Le K.C., Wrapp D., Lee A.G., Liu Y., Chou C.-W., Byrne P.O., Hjorth C.K., Johnson N. V., Ludes-Meyers J., Nguyen A.W., Park J., Wang N., Amengor D., Lavinder J.J., Ippolito G.C., Maynard J.A., Finkelstein I.J., McLellan J.S., 2020. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science eabd0826. 10.1126/science.abd0826 - DOI - PMC - PubMed
-
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H., mRNA-1273 Study Group, 2020. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 383, 1920–1931. 10.1056/NEJMoa2022483 - DOI - PMC - PubMed
-
- Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., Miller J., Schrag S.J., Verani J.R., 2022. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 327, 639–651. 10.1001/jama.2022.0470 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
