This is a preprint.
Baseline levels and longitudinal rates of change in plasma Aβ42/40 among self-identified Black/African American and White individuals
- PMID: 38260384
- PMCID: PMC10802715
- DOI: 10.21203/rs.3.rs-3783571/v1
Baseline levels and longitudinal rates of change in plasma Aβ42/40 among self-identified Black/African American and White individuals
Abstract
Objective: The use of blood-based biomarkers of Alzheimer disease (AD) may facilitate access to biomarker testing of groups that have been historically under-represented in research. We evaluated whether plasma Aβ42/40 has similar or different baseline levels and longitudinal rates of change in participants racialized as Black or White.
Methods: The Study of Race to Understand Alzheimer Biomarkers (SORTOUT-AB) is a multi-center longitudinal study to evaluate for potential differences in AD biomarkers between individuals racialized as Black or White. Plasma samples collected at three AD Research Centers (Washington University, University of Pennsylvania, and University of Alabama-Birmingham) underwent analysis with C2N Diagnostics' PrecivityAD™ blood test for Aβ42 and Aβ40. General linear mixed effects models were used to estimate the baseline levels and rates of longitudinal change for plasma Aβ measures in both racial groups. Analyses also examined whether dementia status, age, sex, education, APOE ε4 carrier status, medical comorbidities, or fasting status modified potential racial differences.
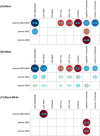
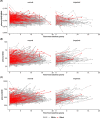
Results: Of the 324 Black and 1,547 White participants, there were 158 Black and 759 White participants with plasma Aβ measures from at least two longitudinal samples over a mean interval of 6.62 years. At baseline, the group of Black participants had lower levels of plasma Aβ40 but similar levels of plasma Aβ42 as compared to the group of White participants. As a result, baseline plasma Aβ42/40 levels were higher in the Black group than the White group, consistent with the Black group having lower levels of amyloid pathology. Racial differences in plasma Aβ42/40 were not modified by age, sex, education, APOE ε4 carrier status, medical conditions (hypertension and diabetes), or fasting status. Despite differences in baseline levels, the Black and White groups had a similar longitudinal rate of change in plasma Aβ42/40.
Interpretation: Black individuals participating in AD research studies had a higher mean level of plasma Aβ42/40, consistent with a lower level of amyloid pathology, which, if confirmed, may imply a lower proportion of Black individuals being eligible for AD clinical trials in which the presence of amyloid is a prerequisite. However, there was no significant racial difference in the rate of change in plasma Aβ42/40, suggesting that amyloid pathology accumulates similarly across racialized groups.
Conflict of interest statement
Declarations Disclosures DW has served as a paid consultant for Eli Lilly, GE Healthcare and Qynapse, and serves on a DSMB for Functional Neuromodulation. ER serves on a data monitoring committee for Eli Lilly. TB participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly and Company, Biogen, Eisai, Janssen, and Roche. DG participates as a site investigator in clinical trials sponsored by Biogen and Janssen. He serves as a consultant to Eisai, Lilly, and Roche. DH and RB co-founded and have equity in C2N Diagnostics. DH serves on the scientific advisory board of C2N Diagnostics, Genentech, Denali, Cajal Neurosciences, and Asteroid. SES has served on advisory boards for Eisai. The other Authors declare no Competing Financial or Non-Financial Interests. This work was supported in part by funding from the National Institutes of Health (grant #AG067505). Washington University has a financial interest in C2N Diagnostics and may financially benefit if the company is successful in marketing its product(s) that is/are related to this research. Additional Declarations: Yes there is potential Competing Interest. Bateman: Washington University (coinventor RJB) submitted the US non-provisional patent application “Blood-Based Methods for Detecting CNS Aβ Deposition” (PCT/US2018/030518). This technology is licensed to C2N Diagnostics and used in the PrecivityAD blood test. Washington University and RJB have equity ownership interest in C2N Diagnostics and receive income based on technology licensed by Washington University to C2N Diagnostics. RJB receives income from C2N Diagnostics for serving on the scientific advisory board. RJB serves on the Roche Gantenerumab Steering Committee as an unpaid member. Benzinger: -All support for the present manuscript; NIH, payments to institution-Grants or contracts from any entity; Siemens, payments to institution-Consulting fees; Biogen, Eli Lilly, Eisai, and Bristol Myers Squibb, payments to me-Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Biogen and Eisai, payments to me -Participation on a Data Safety Monitoring Board or Advisory Board; Eisai, Eli Lilly, Bristol Myers Squibb, payments to me -Receipt of equipment, materials, drugs, medical writing, gifts or other services; Avid Radiopharmaceuticals/Eli Lilly (Technology transfer and precursors for radiopharmaceuticals (18F-Florbetapir, 18F-Flortaucipir) LMI (Technology transfer and precursors for radiopharmaceuticals (18F-Pl-2620) Cerveau / Lantheus (Technology transfer and precursors for radiopharmaceuticals (18F-MK-6240)-Consulting fees; Siemens, unpaid -Participation on a Data Safety Monitoring Board or Advisory Board; Siemens, no payments made -Participation on a Data Safety Monitoring Board or Advisory Board; NIH sponsored/External advisor on several grants, No payments other than travel reimbursement -Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid; ASNR Alzheimer’s and ARIA Study Group, QIBA Amyloid PET Working Group, American College of Radiology/AlzNet Work Group, Alzheimer’s Assoc. Clinical Tau PET Work Group, Unpaid Cruchaga: NIA-Support for research Michael J Fox Foundation–Support for research Alzheimer’s Association-Support for research Alector-Provided consulting fees Circular Genomics–Provided consulting fees; participated in leadership role; provided stock options Somalogics–Supported attendance at ASHG 2022 meeting Geldmacher: receive research funding from Biogen, and Janssen (paid to my institution) I receive consulting fees from Eisai, Lilly, and Roche (paid to me individually). Holtzman: DH co-founded has equity in and serves on the scientific advisory board of C2N Diagnostics. DH serves on the scientific advisory board of Genentech, Denali, Cajal Neurosciences, and Asteroid. Morris: Cure Alzheimer’s Fund Research Strategy Counsel Diverse VCID Observational Study Monitoring Board Barcelona Beta Brain Research Foundation Scientific Advisory Board Roberson: Cure Alzheimer’s Fund Research Strategy Counsel Diverse VCID Observational Study Monitoring Board Barcelona Beta Brain Research Foundation Scientific Advisory Board Schindler: C2N Diagnostics was co-founded by Ors. Randall Bateman and David Holtzman, who are faculty members at Washington University. The PrecivityAD test was developed in the laboratory of Dr. Ranqall Bateman at Washington University and licensed to C2N Diagnostics. Washington University will receive royalties from the PrecivityAD test. Dr. Schindler does not have any interest in C2N Diagnostics and has not received any direct research funding or compensation from C2N Diagnostics. Dr. Schindler has served on advisory boards for Eisai. She has received travel support from the Alzheimer’s Association and USAgainstAlzheimers. Dr. Schindler is an unpaid board member for the Greater Missouri Chapter of the Alz Accociation. Shaw: Leslie M Shaw has served as consultant to: Biogen; Roche, Fujirebio and received research grant support from NIH/NIA(ADNIBiomarker Core PI; PENN ADRC-Biomarker Core co-leader); MJFox Foundation for Parkinson’s Disease Research. Wolk: D.A.W. has served as a paid consultant to Eli Lilly and Qynapse. He serves on a DSMB for Functional Neuromodulation and GSK. He is a site investigator for a clinical trial sponsored by Biogen with funding paid to his institution. Xiong: NIH Grant AG067505 Payment received by Dr. Chengjie Xiong from Diadem, Dr. Chengjie Xiong is part of an FDA Advisory Committee on Imaging Medical Products
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

