This is a preprint.
Heterozygous variants in PLCG1 affect hearing, vision, cardiac, and immune function
- PMID: 38260438
- PMCID: PMC10802640
- DOI: 10.1101/2024.01.08.23300523
Heterozygous variants in PLCG1 affect hearing, vision, cardiac, and immune function
Update in
-
Heterozygous variants in PLCG1 affect hearing, vision, cardiac, and immune function.Elife. 2025 Aug 27;13:RP95887. doi: 10.7554/eLife.95887. Elife. 2025. PMID: 40862571 Free PMC article.
Abstract
Phospholipase C isozymes (PLCs) hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), important signaling molecules involved in many cellular processes including Ca2+ release from the endoplasmic reticulum (ER). PLCG1 encodes the PLCγ1 isozyme that is broadly expressed. Hyperactive somatic mutations of PLCG1 are observed in multiple cancers, but only one germline variant has been reported. Here we describe seven individuals with heterozygous missense variants in PLCG1 [p.(Asp1019Gly), p.(His380Arg), p.(Asp1165Gly), and p.(Leu597Phe)] who present with hearing impairment (5/7), ocular pathology (4/7), cardiac septal defects (3/6), and various immunological issues (5/7). To model these variants in vivo, we generated the analogous variants in the Drosophila ortholog, small wing (sl). We created a null allele sl T2A and assessed its expression pattern. sl is broadly expressed, including wing discs, eye discs, and a subset of neurons and glia. sl T2A mutant flies exhibit wing size reductions, ectopic wing veins, and supernumerary photoreceptors. We document that mutant flies also exhibit a reduced lifespan and age-dependent locomotor defects. Expressing wild-type sl in sl T2A mutant flies rescues the loss-of-function phenotypes, whereas the variants increase lethality. Ectopic expression of an established hyperactive PLCG1 variant, p.(Asp1165His) in the wing pouch causes elevated Ca2+ activity and severe wing phenotypes. These phenotypes are also observed when the p.(Asp1019Gly) or p.(Asp1165Gly) variants are overexpressed in the wing pouch, arguing that these are gain-of-function variants. However, the wing phenotypes associated with p.(His380Arg) or p.(Leu597Phe) overexpression are either mild or only partially penetrant. Our data suggest that the heterozygous missense variants reported here affect protein function differentially and contribute to the clinical features observed in the affected individuals.
Keywords: Drosophila; Phospholipase C; phenotypic heterogeneity.
Conflict of interest statement
Conflict of Interest Statement The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics Laboratories.
Figures

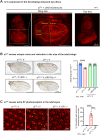
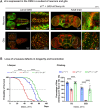

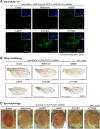

References
-
- Asano S, Maetani Y, Ago Y, Kanematsu T (2022) Phospholipase C-related catalytically inactive protein enhances cisplatin-induced apoptotic cell death. Eur J Pharmacol 933:175273. - PubMed
-
- Baysac K, Sun G, Nakano H, Schmitz EG, Cruz AC, Fisher C, Bailey AC, Group PL-IDW, Mace E, Milner JD, Ombrello MJ (2024) PLCG2-associated immune dysregulation (PLAID) comprises broad and distinct clinical presentations related to functional classes of genetic variants. J Allergy Clin Immunol 153:230–242 - PMC - PubMed
Publication types
Grants and funding
- U24 HG011746/HG/NHGRI NIH HHS/United States
- Z01 OD022005/ImNIH/Intramural NIH HHS/United States
- R24 OD031447/OD/NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- U01 HG007709/HG/NHGRI NIH HHS/United States
- U01 HG010233/HG/NHGRI NIH HHS/United States
- U01 HG011744/HG/NHGRI NIH HHS/United States
- U01 NS134355/NS/NINDS NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
- U01 HG007942/HG/NHGRI NIH HHS/United States
- P50 HD103555/HD/NICHD NIH HHS/United States
- U54 NS093793/NS/NINDS NIH HHS/United States
- R24 OD022005/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
