This is a preprint.
Enhancing radiotherapy response via intratumoral injection of the TLR9 agonist CpG to stimulate CD8 T cells in an autochthonous mouse model of sarcoma
- PMID: 38260522
- PMCID: PMC10802286
- DOI: 10.1101/2024.01.03.573968
Enhancing radiotherapy response via intratumoral injection of the TLR9 agonist CpG to stimulate CD8 T cells in an autochthonous mouse model of sarcoma
Update in
-
Enhancing radiotherapy response via intratumoral injection of a TLR9 agonist in autochthonous murine sarcomas.JCI Insight. 2024 Jun 13;9(14):e178767. doi: 10.1172/jci.insight.178767. JCI Insight. 2024. PMID: 39133651 Free PMC article.
Abstract
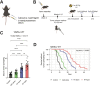
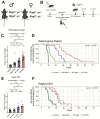
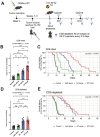
Radiation therapy is frequently used to treat cancers including soft tissue sarcomas. Prior studies established that the toll-like receptor 9 (TLR9) agonist cytosine-phosphate-guanine oligodeoxynucleotide (CpG) enhances the response to radiation therapy (RT) in transplanted tumors, but the mechanism(s) remain unclear. Here, we used CRISPR/Cas9 and the chemical carcinogen 3-methylcholanthrene (MCA) to generate autochthonous soft tissue sarcomas with high tumor mutation burden. Treatment with a single fraction of 20 Gy RT and two doses of CpG significantly enhanced tumor response, which was abrogated by genetic or immunodepletion of CD8+ T cells. To characterize the immune response to RT + CpG, we performed bulk RNA-seq, single-cell RNA-seq, and mass cytometry. Sarcomas treated with 20 Gy and CpG demonstrated increased CD8 T cells expressing markers associated with activation and proliferation, such as Granzyme B, Ki-67, and interferon-γ. CpG + RT also upregulated antigen presentation pathways on myeloid cells. Furthermore, in sarcomas treated with CpG + RT, TCR clonality analysis suggests an increase in clonal T-cell dominance. Collectively, these findings demonstrate that RT + CpG significantly delays tumor growth in a CD8 T cell-dependent manner. These results provide a strong rationale for clinical trials evaluating CpG or other TLR9 agonists with RT in patients with soft tissue sarcoma.
Conflict of interest statement
DGK is a co-founder of Xrad Therapeutics, which is developing radiosensitizers, and serves on the Scientific Advisory Board of Lumicell, which is commercializing intraoperative imaging technology. DGK is a co-inventor on patents for radiosensitizers and an intraoperative imaging device. DGK also receives funding for a clinical trial from a Stand Up To Cancer (SU2C) Catalyst Research Grant with support from Merck. The laboratory of DGK received funding from Xrad Therapeutics, Merck, Bristol-Myers Squibb, but this did not support the research described in this manuscript. DGK received funding from Varian Medical Systems and an antibody to OX-40 from Bristol-Myers Squibb, which were used to support experiments in this manuscript. The other authors have declared that no conflict of interest exists.
Figures




References
-
- Karavasilis V, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112(7):1585–1591. - PubMed
-
- Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–1235. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
