This is a preprint.
Microvilli regulate the release modes of alpha-tectorin to organize the domain-specific matrix architecture of the tectorial membrane
- PMID: 38260557
- PMCID: PMC10802356
- DOI: 10.1101/2024.01.04.574255
Microvilli regulate the release modes of alpha-tectorin to organize the domain-specific matrix architecture of the tectorial membrane
Abstract
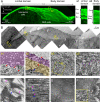
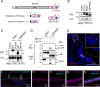
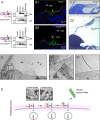
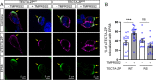
The tectorial membrane (TM) is an apical extracellular matrix (ECM) in the cochlea essential for auditory transduction. The TM exhibits highly ordered domain-specific architecture. Alpha-tectorin/TECTA is a glycosylphosphatidylinositol (GPI)-anchored ECM protein essential for TM organization. Here, we identified that TECTA is released by distinct modes: proteolytic shedding by TMPRSS2 and GPI-anchor-dependent release from the microvillus tip. In the medial/limbal domain, proteolytically shed TECTA forms dense fibers. In the lateral/body domain produced by the supporting cells displaying dense microvilli, the proteolytic shedding restricts TECTA to the microvillus tip and compartmentalizes the collagen-binding site. The tip-localized TECTA, in turn, is released in a GPI-anchor-dependent manner to form collagen-crosslinking fibers, required for maintaining the spacing and parallel organization of collagen fibrils. Overall, we showed that distinct release modes of TECTA determine the domain-specific organization pattern, and the microvillus coordinates the release modes along its membrane to organize the higher-order ECM architecture.
Keywords: ECM; TECTA; TMPRSS2; alpha-tectorin; collagen; extracellular matrix; microvilli; tectorial membrane.
Figures

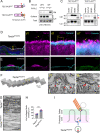
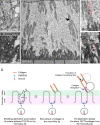
Similar articles
-
Microvilli control the morphogenesis of the tectorial membrane extracellular matrix.Dev Cell. 2025 Mar 10;60(5):679-695.e8. doi: 10.1016/j.devcel.2024.11.011. Epub 2024 Dec 9. Dev Cell. 2025. PMID: 39657673
-
The release of surface-anchored α-tectorin, an apical extracellular matrix protein, mediates tectorial membrane organization.Sci Adv. 2019 Nov 27;5(11):eaay6300. doi: 10.1126/sciadv.aay6300. eCollection 2019 Nov. Sci Adv. 2019. PMID: 31807709 Free PMC article.
-
Characterization of a spontaneous, recessive, missense mutation arising in the Tecta gene.J Assoc Res Otolaryngol. 2008 Jun;9(2):202-14. doi: 10.1007/s10162-008-0116-0. Epub 2008 May 2. J Assoc Res Otolaryngol. 2008. PMID: 18452040 Free PMC article.
-
Structure, Function, and Development of the Tectorial Membrane: An Extracellular Matrix Essential for Hearing.Curr Top Dev Biol. 2018;130:217-244. doi: 10.1016/bs.ctdb.2018.02.006. Epub 2018 Mar 26. Curr Top Dev Biol. 2018. PMID: 29853178 Review.
-
[From gene to disease; DFNA8/12, an autosomal dominant inherited bowl-shaped sensorineural hearing impairment].Ned Tijdschr Geneeskd. 2007 Mar 3;151(9):531-4. Ned Tijdschr Geneeskd. 2007. PMID: 17373394 Review. Dutch.
References
-
- Peixoto C.A., and De Souza W. (1995). Freeze-fracture and deep-etched view of the cuticle of Caenorhabditis elegans. Tissue Cell 27, 561–568. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
