This is a preprint.
HIF1α-glycolysis engages activation-induced cell death to drive IFN-γ induction in hypoxic T cells
- PMID: 38260594
- PMCID: PMC10802708
- DOI: 10.21203/rs.3.rs-3830704/v1
HIF1α-glycolysis engages activation-induced cell death to drive IFN-γ induction in hypoxic T cells
Update in
-
HIF1α-regulated glycolysis promotes activation-induced cell death and IFN-γ induction in hypoxic T cells.Nat Commun. 2024 Oct 30;15(1):9394. doi: 10.1038/s41467-024-53593-8. Nat Commun. 2024. PMID: 39477954 Free PMC article.
Abstract
The role of HIF1α-glycolysis in regulating IFN-γ induction in hypoxic T cells is unknown. Given that hypoxia is a common feature in a wide array of pathophysiological contexts such as tumor and that IFN-γ is instrumental for protective immunity, it is of great significance to gain a clear idea on this. Combining pharmacological and genetic gain-of-function and loss-of-function approaches, we find that HIF1α-glycolysis controls IFN-γ induction in both human and mouse T cells activated under hypoxia. Specific deletion of HIF1α in T cells (HIF1α-/-) and glycolytic inhibition significantly abrogate IFN-γ induction. Conversely, HIF1α stabilization in T cells by hypoxia and VHL deletion (VHL-/-) promotes IFN-γ production. Mechanistically, reduced IFN-γ production in hypoxic HIF1α-/- T cells is due to attenuated activation-induced cell death but not proliferative defect. We further show that depletion of intracellular acetyl-CoA is a key metabolic underlying mechanism. Hypoxic HIF1α-/- T cells are less able to kill tumor cells, and HIF1α-/- tumor-bearing mice are not responsive to immune checkpoint blockade (ICB) therapy, indicating loss of HIF1α in T cells is a major mechanism of therapeutic resistance to ICBs. Importantly, acetate supplementation restores IFN-γ production in hypoxic HIF1α-/- T cells and re-sensitizes HIF1α-/- tumor-bearing mice to ICBs, providing an effective strategy to overcome ICB resistance. Taken together, our results highlight T cell HIF1α-anaerobic glycolysis as a principal mediator of IFN-γ induction and anti-tumor immunity. Considering that acetate supplementation (i.e., glycerol triacetate (GTA)) is approved to treat infants with Canavan disease, we envision a rapid translation of our findings, justifying further testing of GTA as a repurposed medicine for ICB resistance, a pressing unmet medical need.
Keywords: HIF1α; ICB; IFN-γ; T cell; acetate supplementation; anaerobic glycolysis; hypoxia.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures

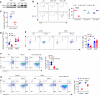
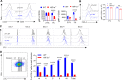

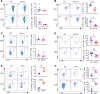

References
-
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A. & Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136, 2348–2357 (1986). - PubMed
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M. & Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155, 1151–1164 (1995). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

