This is a preprint.
Pretransplant Desensitization of Donor-Specific Anti-HLA Antibodies with Plasmapheresis and Immunoglobulin Produces Equivalent Outcomes to Patients with No Donor Specific Antibodies in Haploidentical Hematopoietic Cell Transplant
- PMID: 38260672
- PMCID: PMC10802720
- DOI: 10.21203/rs.3.rs-3832106/v1
Pretransplant Desensitization of Donor-Specific Anti-HLA Antibodies with Plasmapheresis and Immunoglobulin Produces Equivalent Outcomes to Patients with No Donor Specific Antibodies in Haploidentical Hematopoietic Cell Transplant
Update in
-
Pretransplant desensitization of donor-specific anti-HLA antibodies with plasmapheresis and immunoglobulin produces equivalent outcomes to patients with no donor specific antibodies in haploidentical hematopoietic cell transplant.Leuk Lymphoma. 2024 Dec;65(12):1811-1819. doi: 10.1080/10428194.2024.2376172. Epub 2024 Jul 11. Leuk Lymphoma. 2024. PMID: 38990135
Abstract
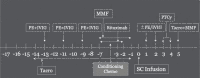
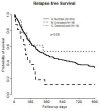
In patients requiring haploidentical hematopoietic cell transplant (haplo-HCT), the presence of donor specific anti-HLA antibodies (DSAs) is associated with high rates of primary graft failure and poor overall survival (OS). There is limited data regarding the effect of desensitization. Adult patients undergoing haplo-HCT at Washington University School of Medicine from 2009-2021 were identified. Patients were divided into three cohorts: no DSA, untreated DSA or treated DSA. DSA testing was performed. Desensitization therapy using plasmapheresis and IVIg (immunoglobulin) was performed. We retrospectively identified 304 patients for study inclusion. 14 of 30 patients with DSAs underwent desensitization. By day +2, 57% of patients cleared all DSAs. OS was expectedly worse in patients with untreated DSAs. There were similar results between treated DSA and patients without DSA (OS median: control: 352 days vs. treated: 1331 days vs. untreated: 137 days, p = 0.02). RFS was also significantly different between the groups however with similar RFS in treated DSA and control groups (RFS median: control: 248 vs. treated: 322 v. untreated: 119, p = 0.03). Desensitization before haplo-HCT produces similar outcomes to patients without DSAs. While the optimal desensitization protocol has not been established, all patients received a backbone of plasmapheresis and immunoglobulin.
Conflict of interest statement
Declarations Conflict of Interest: The authors declare that they have no conflicts relating to this manuscript. Additional Declarations: The authors have declared there is NO conflict of interest to disclose.
Figures



References
-
- Takami A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int J Hematol. 2018. May;107(5):513–8. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

