Rapid and reliable diagnosis of Moraxella catarrhalis infection using loop-mediated isothermal amplification-based testing
- PMID: 38260738
- PMCID: PMC10800902
- DOI: 10.3389/fbioe.2023.1330047
Rapid and reliable diagnosis of Moraxella catarrhalis infection using loop-mediated isothermal amplification-based testing
Abstract
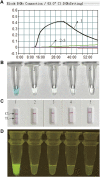
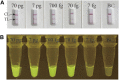
Moraxella catarrhalis (M. catarrhalis) was an important pathogen closely associated with respiratory tract infections. We employed the loop-mediated isothermal amplification (LAMP) coupled with nanoparticle-based lateral flow biosensor (LFB) and fluorescence testing technique for formulating two diagnostic methods for M. catarrhalis detection, termed M. catarrhalis-LAMP-LFB assay and M. catarrhalis-LAMP-FRT, respectively. The M. catarrhalis-LAMP-LFB system incorporated the use of biotin-14-dCTP and a forward loop primer (LF) with a hapten at the 5' end. This design in LAMP reaction enabled the production of double-labeled products that could be effectively analyzed using the lateral flow biosensor (LFB). For the M. catarrhalis-LAMP-FRT assay, the LF was modified with a sequence at 5' end, and a fluorophore, as well as a black hole quencher, were strategically labeled at the 5' end and within the middle of the new LF. The restriction endonuclease Nb.BsrDI could accurately recognize and cleave the newly synthesized double-strand terminal sequences, resulting in the separation of the fluorophore from the black hole quencher and releasing fluorescence signals. Both assays have been proven to be highly sensitive and specific, capable of detecting genomic DNA of M. catarrhalis at concentrations as low as 70 fg, with no cross-reactivity observed with non-M. catarrhalis pathogens. Furthermore, both methods successfully identified M. catarrhalis in all clinical samples within 1 h that were confirmed positive by real-time PCR, exhibiting superior sensitivity than conventional culture methods. Herein, the newly developed two LAMP-based assays were rapid and reliable for M. catarrhalis detection and hold significant promise for deployment in point-of-care (POC) settings.
Keywords: Moraxella catarrhalis; loop-mediated isothermal amplification; nanoparticle-based lateral flow biosensor; restriction endonuclease; visual detection.
Copyright © 2024 Xiao, Zhou, Huang, Fu, Jia, Sun, Xu, Wang, Yu and Meng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The rapid and visual detection of methicillin-susceptible and methicillin-resistant Staphylococcus aureus using multiplex loop-mediated isothermal amplification linked to a nanoparticle-based lateral flow biosensor.Antimicrob Resist Infect Control. 2020 Jul 17;9(1):111. doi: 10.1186/s13756-020-00774-x. Antimicrob Resist Infect Control. 2020. PMID: 32680560 Free PMC article.
-
Development of loop-mediated isothermal amplification coupled with nanoparticle-based lateral flow biosensor assay for Mycoplasma pneumoniae detection.AMB Express. 2019 Dec 5;9(1):196. doi: 10.1186/s13568-019-0921-3. AMB Express. 2019. PMID: 31807946 Free PMC article.
-
Development and Preliminary Application of Multiplex Loop-Mediated Isothermal Amplification Coupled With Lateral Flow Biosensor for Detection of Mycobacterium tuberculosis Complex.Front Cell Infect Microbiol. 2021 Apr 27;11:666492. doi: 10.3389/fcimb.2021.666492. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33987108 Free PMC article.
-
Loop-Mediated Isothermal Amplification Coupled With Nanoparticle-Based Lateral Biosensor for Rapid, Sensitive, and Specific Detection of Bordetella pertussis.Front Bioeng Biotechnol. 2022 Feb 8;9:797957. doi: 10.3389/fbioe.2021.797957. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35211469 Free PMC article. Review.
-
Lateral flow biosensor combined with loop-mediated isothermal amplification for simple, rapid, sensitive, and reliable detection of Brucella spp.Infect Drug Resist. 2019 Jul 30;12:2343-2353. doi: 10.2147/IDR.S211644. eCollection 2019. Infect Drug Resist. 2019. PMID: 31440069 Free PMC article. Review.
Cited by
-
Detection of Toxoplasma gondii in Brain Tissues of Pet Birds with Neurological Symptoms Using Loop-Mediated Isothermal Amplification (LAMP) and PCR.Acta Parasitol. 2025 Jan 24;70(1):40. doi: 10.1007/s11686-024-00983-z. Acta Parasitol. 2025. PMID: 39853511
References
LinkOut - more resources
Full Text Sources

