Elimination and penetration of amikacin into urine in patients with decreased glomerular filtration rate
- PMID: 38260825
- PMCID: PMC10802929
- DOI: 10.1093/ckj/sfae002
Elimination and penetration of amikacin into urine in patients with decreased glomerular filtration rate
Abstract
Background: Amikacin monotherapy is recommended for urinary tract infection (UTI) treatment with multi-resistant pathogens. Even though amikacin efficacy in the treatment of UTIs is dependent on its urinary concentration, there are no robust data proving that sufficiently high urinary concentration is reached in patients with reduced glomerular filtration rate (GFR).
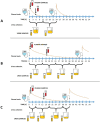
Methods: A prospective study to monitor amikacin penetration into urine of 70 patients [40 males, median (interquartile range) age 70 (65-79) years] with different levels of glomerular filtration decline, including patients treated by dialysis, was conducted. The bactericidal efficacy of amikacin in urine samples has been evaluated.
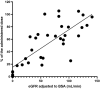
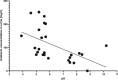
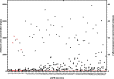
Results: Patients with estimated GFR (eGFR) <30 mL/min had significantly lower median amikacin urinary concentration than patients with eGFR >30 mL/min (89.75 vs 186.0 mg/L, P < .0001; 200.5 vs 830.0 mg/L, P < .0001; and 126.0 vs 408.0 mg/L, P < .0001 for minimal, maximal and minimal together with maximal concentrations, respectively). The amount of amikacin eliminated in the first 10-13 h after dose administration was dependent on eGFR (r2 = 0.6144, P < .0001). The urinary concentration of amikacin in patients treated by dialysis was indirectly proportional to pH of urine. The plasma concentrations of amikacin did not correlate with urinary levels in patients in either of the GFR categories. Microbiological evaluation showed that the critical urinary concentration for efficacy of amikacin during UTI monotherapy in patients treated by dialysis is 100 mg/L. We found that 4 out of 11 patients treated by dialysis did not reach this level during the treatment.
Conclusion: Systemic administration of amikacin monotherapy in patients treated by dialysis is questionable as the concentrations of amikacin in their urine are often below the threshold of effectivity. Amikacin plasma concentrations are not a major determinant of amikacin concentration in urine, therefore pulse dosing is neither necessary nor safe in patients treated by dialysis, and may cause undesirable toxicity.
Keywords: UTI; aminoglycosides; dialysis; kidney impairment; pharmacokinetics.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The authors declare they do not have any conflict of interest regarding this article.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

