Functional candesartan loaded lipid nanoparticles for the control of diabetes-associated stroke: In vitro and in vivo studies
- PMID: 38260917
- PMCID: PMC10801309
- DOI: 10.1016/j.ijpx.2023.100227
Functional candesartan loaded lipid nanoparticles for the control of diabetes-associated stroke: In vitro and in vivo studies
Abstract
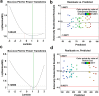
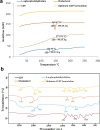
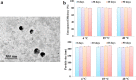
Diabetes mellitus is a metabolic disease that raises the odds of developing stroke. Candesartan has been used to prevent stroke due to its inhibitory effects on blood pressure, angiogenesis, oxidative damage, and apoptosis. However, oral candesartan has very limited bioavailability and efficacy due to its weak solubility and slow release. The study aimed to develop a nasal formulation of candesartan-loaded liposomes containing ethanol and propylene glycol (CLEP) to improve candesartan's delivery, release, permeation, and efficacy as a potential diabetes-associated stroke treatment. Using design expert software, different CLEP formulations were prepared and evaluated in vitro to identify the optimum formulation, which. The selected optimum formulation composed of 3.3% phospholipid, 10% ethanol, and 15% propylene glycol significantly increased the release and permeation of candesartan relative to free candesartan by a factor of 1.52 and 1.47, respectively. The optimum formulation significantly reduced the infarction after stroke in rats; decreased flexion, spontaneous motor activity, and time spent in the target quadrant by 70%, 64.71%, and 92.31%, respectively, and enhanced grip strength by a ratio of 2.3. Therefore, nasal administration of the CLEP formulation could be a potential diabetes-associated stroke treatment.
Keywords: Candesartan; Diabetes mellitus; Ethanol; Liposomes; Propylene glycol; Stroke.
© 2023 The Author(s). Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Basmah nasser reports financial support was provided by King Saud University. Randa Zaki reports a relationship with Prince Sattam bin Abdulaziz University that includes: employment and funding grants.
Figures






References
-
- Abdelbary A.A., AbouGhaly M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015;485(1–2):235–243. doi: 10.1016/j.ijpharm.2015.03.020. - DOI - PubMed
LinkOut - more resources
Full Text Sources

