Clinical development of a blood biomarker using apolipoprotein-A2 isoforms for early detection of pancreatic cancer
- PMID: 38261000
- PMCID: PMC10904523
- DOI: 10.1007/s00535-023-02072-w
Clinical development of a blood biomarker using apolipoprotein-A2 isoforms for early detection of pancreatic cancer
Abstract
Background: We have previously reported apolipoprotein A2-isoforms (apoA2-is) as candidate plasma biomarkers for early-stage pancreatic cancer. The aim of this study was the clinical development of apoA2-is.
Methods: We established a new enzyme-linked immunosorbent sandwich assay for apoA2-is under the Japanese medical device Quality Management System requirements and performed in vitro diagnostic tests with prespecified end points using 2732 plasma samples. The clinical equivalence and significance of apoA2-is were compared with CA19-9.
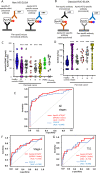
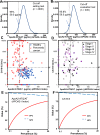
Results: The point estimate of the area under the curve to distinguish between pancreatic cancer (n = 106) and healthy controls (n = 106) was higher for apoA2-ATQ/AT [0.879, 95% confidence interval (CI): 0.832-0.925] than for CA19-9 (0.849, 95% CI 0.793-0.905) and achieved the primary end point. The cutoff apoA2-ATQ/AT of 59.5 μg/mL was defined based on a specificity of 95% in 2000 healthy samples, and the reliability of specificities was confirmed in two independent healthy cohorts as 95.3% (n = 106, 95% CI 89.4-98.0%) and 95.8% (n = 400, 95% CI 93.3-97.3%). The sensitivities of apoA2-ATQ/AT for detecting both stage I (47.4%) and I/II (50%) pancreatic cancers were higher than those of CA19-9 (36.8% and 46.7%, respectively). The combination of apoA2-ATQ/AT (cutoff, 59.5 μg/mL) and CA19-9 (37 U/mL) increased the sensitivity for pancreatic cancer to 87.7% compared with 69.8% for CA19-9 alone. The clinical performance of apoA2-is was blindly confirmed by the National Cancer Institute Early Detection Research Network.
Conclusions: The clinical performance of ApoA2-ATQ/AT as a blood biomarker is equivalent to or better than that of CA19-9.
Keywords: Apolipoprotein A2-isoform; Blood biomarker; Carbohydrate antigen 19-9 (CA19-9); Early detection of pancreatic cancer.
© 2024. The Author(s).
Conflict of interest statement
Kazufumi Honda and Kengo Nagashima are advisers to Toray Industries, Inc. Kazufumi Honda, Giman Jung, and Michimoto Kobayashi are inventors with patent filings for apoA2-i. None of the other authors have any conflicts of interest to declare.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

