Effect of CO2 Concentrations on Entomopathogen Fitness and Insect-Pathogen Interactions
- PMID: 38261023
- PMCID: PMC10805855
- DOI: 10.1007/s00248-024-02347-6
Effect of CO2 Concentrations on Entomopathogen Fitness and Insect-Pathogen Interactions
Abstract
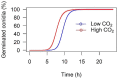
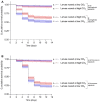
Numerous insect species and their associated microbial pathogens are exposed to elevated CO2 concentrations in both artificial and natural environments. However, the impacts of elevated CO2 on the fitness of these pathogens and the susceptibility of insects to pathogen infections are not well understood. The yellow mealworm, Tenebrio molitor, is commonly produced for food and feed purposes in mass-rearing systems, which increases risk of pathogen infections. Additionally, entomopathogens are used to control T. molitor, which is also a pest of stored grains. It is therefore important to understand how elevated CO2 may affect both the pathogen directly and impact on host-pathogen interactions. We demonstrate that elevated CO2 concentrations reduced the viability and persistence of the spores of the bacterial pathogen Bacillus thuringiensis. In contrast, conidia of the fungal pathogen Metarhizium brunneum germinated faster under elevated CO2. Pre-exposure of the two pathogens to elevated CO2 prior to host infection did not affect the survival probability of T. molitor larvae. However, larvae reared at elevated CO2 concentrations were less susceptible to both pathogens compared to larvae reared at ambient CO2 concentrations. Our findings indicate that whilst elevated CO2 concentrations may be beneficial in reducing host susceptibility in mass-rearing systems, they may potentially reduce the efficacy of the tested entomopathogens when used as biological control agents of T. molitor larvae. We conclude that CO2 concentrations should be carefully selected and monitored as an additional environmental factor in laboratory experiments investigating insect-pathogen interactions.
Keywords: Bacillus thuringiensis; Biocontrol; Host-pathogen Interactions; Insect Culture; Metarhizium brunneum; Tenebrio molitor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Keeling RF, Keeling CD. 2017. Scripps CO2 Program Data UC San. - DOI
-
- Howe JA, Smith AP. The soil habitat. In: Gentry TJ, Fuhrmann JJ, Zuberer DA, editors. Principles and applications of soil microbiology. 3. Amsterdam: Elsevier; 2021. pp. 23–55.
-
- Bollazzi M, Forti LC, Roces F. Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insectes Sociaux. 2012;59:487–498. doi: 10.1007/s00040-012-0243-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

