Virus-induced brain pathology and the neuroinflammation-inflammation continuum: the neurochemists view
- PMID: 38261034
- PMCID: PMC11608394
- DOI: 10.1007/s00702-023-02723-5
Virus-induced brain pathology and the neuroinflammation-inflammation continuum: the neurochemists view
Abstract
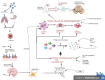
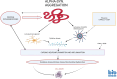
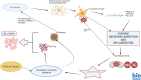
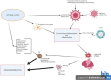
Fascinatingly, an abundance of recent studies has subscribed to the importance of cytotoxic immune mechanisms that appear to increase the risk/trigger for many progressive neurodegenerative disorders, including Parkinson's disease (PD), Alzheimer's disease (AD), amyotrophic lateral sclerosis, and multiple sclerosis. Events associated with the neuroinflammatory cascades, such as ageing, immunologic dysfunction, and eventually disruption of the blood-brain barrier and the "cytokine storm", appear to be orchestrated mainly through the activation of microglial cells and communication with the neurons. The inflammatory processes prompt cellular protein dyshomeostasis. Parkinson's and Alzheimer's disease share a common feature marked by characteristic pathological hallmarks of abnormal neuronal protein accumulation. These Lewy bodies contain misfolded α-synuclein aggregates in PD or in the case of AD, they are Aβ deposits and tau-containing neurofibrillary tangles. Subsequently, these abnormal protein aggregates further elicit neurotoxic processes and events which contribute to the onset of neurodegeneration and to its progression including aggravation of neuroinflammation. However, there is a caveat for exclusively linking neuroinflammation with neurodegeneration, since it's highly unlikely that immune dysregulation is the only factor that contributes to the manifestation of many of these neurodegenerative disorders. It is unquestionably a complex interaction with other factors such as genetics, age, and environment. This endorses the "multiple hit hypothesis". Consequently, if the host has a genetic susceptibility coupled to an age-related weakened immune system, this makes them more susceptible to the virus/bacteria-related infection. This may trigger the onset of chronic cytotoxic neuroinflammatory processes leading to protein dyshomeostasis and accumulation, and finally, these events lead to neuronal destruction. Here, we differentiate "neuroinflammation" and "inflammation" with regard to the involvement of the blood-brain barrier, which seems to be intact in the case of neuroinflammation but defect in the case of inflammation. There is a neuroinflammation-inflammation continuum with regard to virus-induced brain affection. Therefore, we propose a staging of this process, which might be further developed by adding blood- and CSF parameters, their stage-dependent composition and stage-dependent severeness grade. If so, this might be suitable to optimise therapeutic strategies to fight brain neuroinflammation in its beginning and avoid inflammation at all.
Keywords: Neurodegeneration; Alzheimer’s disease; Cytotoxicity; Immunology; Inflammation; Microglia and neuroinflammation; Parkinson’s disease; Viruses.
© 2024. The Author(s).
Figures
Similar articles
-
Mitochondrial Dysfunction is a Crucial Immune Checkpoint for Neuroinflammation and Neurodegeneration: mtDAMPs in Focus.Mol Neurobiol. 2025 Jun;62(6):6715-6747. doi: 10.1007/s12035-024-04412-0. Epub 2024 Aug 8. Mol Neurobiol. 2025. PMID: 39115673 Review.
-
Microglia-driven inflammation induces progressive tauopathies and synucleinopathies.Exp Mol Med. 2025 May;57(5):1017-1031. doi: 10.1038/s12276-025-01450-z. Epub 2025 May 1. Exp Mol Med. 2025. PMID: 40307569 Free PMC article.
-
Chitinase-3-like-1: a multifaceted player in neuroinflammation and degenerative pathologies with therapeutic implications.Mol Neurodegener. 2025 Jan 18;20(1):7. doi: 10.1186/s13024-025-00801-8. Mol Neurodegener. 2025. PMID: 39827337 Free PMC article. Review.
-
The Role of Microglia in Retinal Neurodegeneration: Alzheimer's Disease, Parkinson, and Glaucoma.Front Aging Neurosci. 2017 Jul 6;9:214. doi: 10.3389/fnagi.2017.00214. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28729832 Free PMC article. Review.
-
Constitutively active STING causes neuroinflammation and degeneration of dopaminergic neurons in mice.Elife. 2022 Oct 31;11:e81943. doi: 10.7554/eLife.81943. Elife. 2022. PMID: 36314770 Free PMC article.
Cited by
-
Unraveling the complex interplay: immunopathology and immune evasion strategies of alphaviruses with emphasis on neurological implications.Front Cell Infect Microbiol. 2024 Aug 15;14:1421571. doi: 10.3389/fcimb.2024.1421571. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39211797 Free PMC article. Review.
-
Investigating cuproptosis and mitochondrial dysfunction in brain cells: uncovering novel mechanisms and biomarkers for Parkinson's disease.Metab Brain Dis. 2025 Mar 12;40(3):144. doi: 10.1007/s11011-025-01574-1. Metab Brain Dis. 2025. PMID: 40072691
-
Postencephalitic Parkinsonism: Unique Pathological and Clinical Features-Preliminary Data.Cells. 2024 Sep 10;13(18):1511. doi: 10.3390/cells13181511. Cells. 2024. PMID: 39329695 Free PMC article.
-
Metabolic Dysfunction in Parkinson's Disease: Unraveling the Glucose-Lipid Connection.Biomedicines. 2024 Dec 13;12(12):2841. doi: 10.3390/biomedicines12122841. Biomedicines. 2024. PMID: 39767747 Free PMC article. Review.
-
The dual role of microglia in Alzheimer's disease: from immune regulation to pathological progression.Front Aging Neurosci. 2025 Mar 27;17:1554398. doi: 10.3389/fnagi.2025.1554398. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40212564 Free PMC article. Review.
References
-
- Albornoz EA, Amarilla AA, Modhiran N, Parker S, Li XX, Wijesundara DK, Aguado J, Zamora AP, McMillan CLD, Liang B, Peng NYG, Sng JDJ, Saima FT, Fung JN, Lee JD, Paramitha D, Parry R, Avumegah MS, Isaacs A, Lo MW, Miranda-Chacon Z, Bradshaw D, Salinas-Rebolledo C, Rajapakse NW, Wolvetang EJ, Munro TP, Rojas-Fernandez A, Young PR, Stacey KJ, Khromykh AA, Chappell KJ, Watterson D, Woodruff TM (2022) SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol Psychiatry. 10.1038/s41380-022-01831-0 - PMC - PubMed
-
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR (1995) An english translation of Alzheimer’s 1907 Paper, ‘Über Eine Eigenartige Erkankung Der Hirnrinde.’ Clinical Anatomy (new York, NY) 8(6):429–431. 10.1002/ca.980080612 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

