Canine Mucosal Artificial Colon: development of a new colonic in vitro model adapted to dog sizes
- PMID: 38261090
- PMCID: PMC10806056
- DOI: 10.1007/s00253-023-12987-2
Canine Mucosal Artificial Colon: development of a new colonic in vitro model adapted to dog sizes
Abstract
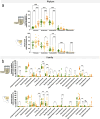
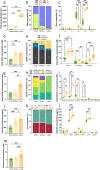
Differences in dog breed sizes are an important determinant of variations in digestive physiology, mainly related to the large intestine. In vitro gut models are increasingly used as alternatives to animal experiments for technical, cost, societal, and regulatory reasons. Up to now, only one in vitro model of the canine colon incorporates the dynamics of different canine gut regions, yet no adaptations exist to reproduce size-related digestive parameters. To address this limitation, we developed a new model of the canine colon, the CANIne Mucosal ARtificial COLon (CANIM-ARCOL), simulating main physiochemical (pH, transit time, anaerobiosis), nutritional (ileal effluent composition), and microbial (lumen and mucus-associated microbiota) parameters of this ecosystem and adapted to three dog sizes (i.e., small under 10 kg, medium 10-30 kg, and large over 30 kg). To validate the new model regarding microbiota composition and activities, in vitro fermentations were performed in bioreactors inoculated with stools from 13 dogs (4 small, 5 medium, and 4 large). After a stabilization period, microbiota profiles clearly clustered depending on dog size. Bacteroidota and Firmicutes abundances were positively correlated with dog size both in vitro and in vivo, while opposite trends were observed for Actinobacteria and Proteobacteria. As observed in vivo, microbial activity also increased with dog size in vitro, as evidenced from gas production, short-chain fatty acids, ammonia, and bile acid dehydroxylation. In line with the 3R regulation, CANIM-ARCOL could be a relevant platform to assess bilateral interactions between food and pharma compounds and gut microbiota, capturing inter-individual or breed variabilities. KEY POINTS: • CANIM-ARCOL integrates main canine physicochemical and microbial colonic parameters • Gut microbiota associated to different dog sizes is accurately maintained in vitro • The model can help to move toward personalized approach considering dog body weight.
Keywords: Body weight; Breed; In vitro gut model; Large intestine; Microbiota; Mucus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Large intestinal nutritional and physicochemical parameters from different dog sizes reshape canine microbiota structure and functions in vitro.Bioengineered. 2024 Dec;15(1):2325713. doi: 10.1080/21655979.2024.2325713. Epub 2024 Mar 12. Bioengineered. 2024. PMID: 38471972 Free PMC article.
-
Development of a new antibiotic-induced dysbiosis model of the canine colonic microbiota.Int J Antimicrob Agents. 2024 Apr;63(4):107102. doi: 10.1016/j.ijantimicag.2024.107102. Epub 2024 Feb 5. Int J Antimicrob Agents. 2024. PMID: 38325721
-
A child is not an adult: development of a new in vitro model of the toddler colon.Appl Microbiol Biotechnol. 2022 Nov;106(21):7315-7336. doi: 10.1007/s00253-022-12199-0. Epub 2022 Oct 7. Appl Microbiol Biotechnol. 2022. PMID: 36202936 Review.
-
A specific blend of prebiotics and postbiotics improved the gut microbiome of dogs with soft stools in the in vitro Simulator of the Canine Intestinal Microbial Ecosystem.J Anim Sci. 2025 Jan 4;103:skaf056. doi: 10.1093/jas/skaf056. J Anim Sci. 2025. PMID: 40036370 Free PMC article.
-
From Chihuahua to Saint-Bernard: how did digestion and microbiota evolve with dog sizes.Int J Biol Sci. 2022 Aug 1;18(13):5086-5102. doi: 10.7150/ijbs.72770. eCollection 2022. Int J Biol Sci. 2022. PMID: 35982892 Free PMC article. Review.
Cited by
-
Large intestinal nutritional and physicochemical parameters from different dog sizes reshape canine microbiota structure and functions in vitro.Bioengineered. 2024 Dec;15(1):2325713. doi: 10.1080/21655979.2024.2325713. Epub 2024 Mar 12. Bioengineered. 2024. PMID: 38471972 Free PMC article.
-
Cultivating complexity: Advancements in establishing in vitro models for the mucus-adhering gut microbiota.Microb Biotechnol. 2024 Oct;17(10):e70036. doi: 10.1111/1751-7915.70036. Microb Biotechnol. 2024. PMID: 39435730 Free PMC article. Review.
-
Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance.Metabolites. 2024 Mar 22;14(4):178. doi: 10.3390/metabo14040178. Metabolites. 2024. PMID: 38668306 Free PMC article. Review.
-
Gut microbial dysbiosis associated to diarrheic irritable bowel syndrome can be efficiently simulated in the Mucosal ARtificial COLon (M-ARCOL).Bioengineered. 2025 Dec;16(1):2458362. doi: 10.1080/21655979.2025.2458362. Epub 2025 Feb 4. Bioengineered. 2025. PMID: 39902883 Free PMC article.
References
-
- Algya KM, Cross T-WL, Leuck KN, Kastner ME, Baba T, Lye L, de Godoy MRC, Swanson KS (2018) Apparent total-tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked, and raw diets. J Anim Sci 96:3670–3683. 10.1093/jas/sky235 - PMC - PubMed
-
- AlShawaqfeh MK, Wajid B, Minamoto Y, Markel M, Lidbury JA, Steiner JM, Serpedin E, Suchodolski JS (2017) A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol Ecol 93:fix136. 10.1093/femsec/fix136 - PubMed
-
- Apper E, Privet L, Taminiau B, Le Bourgot C, Svilar L, Martin J-C, Diez M (2020) Relationships between gut microbiota, metabolome, body weight, and glucose homeostasis of obese dogs fed with diets differing in prebiotic and protein content. Microorganisms 8:513. 10.3390/microorganisms8040513 - PMC - PubMed
-
- Axelsson E, Ratnakumar A, Arendt M-L, Maqbool K, Webster MT, Perloski M, Liberg O, Arnemo JM, Hedhammar Å, Lindblad-Toh K (2013) The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495:360–364. 10.1038/nature11837 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

