AOTMiT reimbursement recommendations compared to other HTA agencies
- PMID: 38261131
- PMCID: PMC11442500
- DOI: 10.1007/s10198-023-01655-x
AOTMiT reimbursement recommendations compared to other HTA agencies
Erratum in
-
Correction: AOTMiT reimbursement recommendations compared to other HTA agencies.Eur J Health Econ. 2025 Feb;26(1):143-146. doi: 10.1007/s10198-024-01720-z. Epub 2024 Oct 26. Eur J Health Econ. 2025. PMID: 39460843 Free PMC article. No abstract available.
Abstract
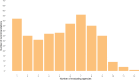
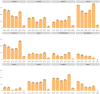
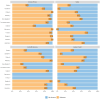
Our objective was to compare AOTMiT (Polish: Agencja Oceny Technologii Medycznych i Taryfikacji) recommendations to other HTA (Health Technology Assessment) agencies for newly registered drugs and new registration indications issued by the European Medicines Agency between 2014 and 2019. The study aims to assess the consistency and justifications of AOTMiT recommendations compared to that of other HTA agencies in 11 countries. A total of 2496 reimbursement recommendations published by 12 HTA agencies for 464 medicinal products and 525 indications were analyzed. Our analysis confirmed that the Polish AOTMiT agency seems to bear the closest resemblance to the corresponding HTA agencies from Canada (CADTH) and New Zealand (PHARMAC), when it comes to the outcome of HTA recommendations (positive or negative). Poland had a general scheme for justifying recommendations, similar to that of Ireland-four aspects (i.e., clinical efficacy, safety profile, cost-effectiveness, and impact on the payer's budget) are important for Poland when formulating the final decision. Compared to other countries, Poland shows a noticeably different pattern of justifying reimbursement recommendations, as revealed primarily in terms of budget impact and somewhat less so for cost-effectiveness rationales.
Keywords: AHTAPol; AOTMiT; HTA agencies; Reimbursement recommendations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Time from approval to reimbursement recommendations in healthcare systems with centralized HTA processes. Focus on the Polish HTA agency.Expert Rev Pharmacoecon Outcomes Res. 2025 Feb;25(2):265-273. doi: 10.1080/14737167.2024.2416240. Epub 2024 Oct 21. Expert Rev Pharmacoecon Outcomes Res. 2025. PMID: 39431604
-
Relating Health Technology Assessment recommendations and reimbursement decisions in Poland in years 2012-2014, a retrospective analysis.Health Policy. 2016 Nov;120(11):1240-1248. doi: 10.1016/j.healthpol.2016.09.021. Epub 2016 Oct 11. Health Policy. 2016. PMID: 28029415
-
A DECADE OF HEALTH TECHNOLOGY ASSESSMENT IN POLAND.Int J Technol Assess Health Care. 2017 Jan;33(3):350-357. doi: 10.1017/S0266462317000563. Epub 2017 Jul 19. Int J Technol Assess Health Care. 2017. PMID: 28720170
-
Reimbursement decision-making system in Poland systematically compared to other countries.Front Pharmacol. 2023 Oct 13;14:1153680. doi: 10.3389/fphar.2023.1153680. eCollection 2023. Front Pharmacol. 2023. PMID: 37900165 Free PMC article.
-
Comparative Assessment of Reimbursement Recommendations by NICE and HAS for Oncology New Medicines Indicated for the Treatment of Solid Tumors from 2015 to 2021.Med Decis Making. 2023 Oct-Nov;43(7-8):961-972. doi: 10.1177/0272989X231188073. Epub 2023 Jul 22. Med Decis Making. 2023. PMID: 37480275 Review.
Cited by
-
Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe.J Mark Access Health Policy. 2025 May 26;13(2):24. doi: 10.3390/jmahp13020024. eCollection 2025 Jun. J Mark Access Health Policy. 2025. PMID: 40568380 Free PMC article.
References
-
- Gozzo, L., Paterson, K., Wong, O., Megerlin, F., Geldmacher, J., Popoli, P., Jommi, C., Fricke, F.-U., De Solà-Morales, O., Kamae, I., Rasi, G., Drago, F.: Towards a European harmonization of health technology assessment recommendations executive paper of European regulatory conference focused on the EU commission proposal to harmonize HTA. Front. Drug. Saf. Regul. 2, 970661 (2022). 10.3389/fdsfr.2022.970661
-
- Jahnz-Różyk, K., Kawalec, P., Malinowski, K., Czok, K.: Drug policy in Poland. Value Health Reg Issues. 13, 23–26 (2017). 10.1016/j.vhri.2017.07.001 - PubMed
-
- Kawalec, P., Sagan, A., Stawowczyk, E., Kowalska-Bobko, I., Mokrzycka, A.: Implementation of the 2011 reimbursement Act in Poland: desired and undesired effects of the changes in reimbursement policy. Health Policy 120(4), 356–361 (2016). 10.1016/j.healthpol.2016.02.010 - PubMed
-
- Kolasa, K., Wasiak, R.: Health technology assessment in Poland and Scotland: comparison of process and decisions. Int. J. Technol. Assess. Health Care 28(1), 70–76 (2012). 10.1017/S0266462311000699 - PubMed
-
- Wüller, H., Sowada, C., Bochenek, T.: Comparison between processes of HTA, pharmaceutical pricing and reimbursement, and their transparency in Germany and Poland. Zeszyty Naukowe Ochrony Zdrowia, Zdrowie Publiczne I Zarządzanie 13(1), 102–108 (2015). 10.4467/20842627OZ.15.010.4123
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

