Histologic and Clinical Factors Associated with Kidney Outcomes in IgA Vasculitis Nephritis
- PMID: 38261310
- PMCID: PMC11020428
- DOI: 10.2215/CJN.0000000000000398
Histologic and Clinical Factors Associated with Kidney Outcomes in IgA Vasculitis Nephritis
Abstract
Background: Nephritis is a common manifestation of IgA vasculitis and is morphologically indistinguishable from IgA nephropathy. While MEST-C scores are predictive of kidney outcomes in IgA nephropathy, their value in IgA vasculitis nephritis has not been investigated in large multiethnic cohorts.
Methods: Biopsies from 262 children and 99 adults with IgA vasculitis nephritis ( N =361) from 23 centers in North America, Europe, and Asia were independently scored by three pathologists. MEST-C scores were assessed for correlation with eGFR/proteinuria at biopsy. Because most patients ( N =309, 86%) received immunosuppression, risk factors for outcomes were evaluated in this group using latent class mixed models to identify classes of eGFR trajectories over a median follow-up of 2.7 years (interquartile range, 1.2-5.1). Clinical and histologic parameters associated with each class were determined using logistic regression.
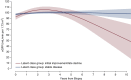
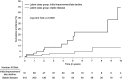
Results: M, E, T, and C scores were correlated with either eGFR or proteinuria at biopsy. Two classes were identified by latent class mixed model, one with initial improvement in eGFR followed by a late decline (class 1, N =91) and another with stable eGFR (class 2, N =218). Class 1 was associated with a higher risk of an established kidney outcome (time to ≥30% decline in eGFR or kidney failure; hazard ratio, 5.84; 95% confidence interval, 2.37 to 14.4). Among MEST-C scores, only E1 was associated with class 1 by multivariable analysis. Other factors associated with class 1 were age 18 years and younger, male sex, lower eGFR at biopsy, and extrarenal noncutaneous disease. Fibrous crescents without active changes were associated with class 2.
Conclusions: Kidney outcome in patients with biopsied IgA vasculitis nephritis treated with immunosuppression was determined by clinical risk factors and endocapillary hypercellularity (E1) and fibrous crescents, which are features that are not part of the International Study of Diseases of Children classification.
Copyright © 2024 by the American Society of Nephrology.
Conflict of interest statement
C.E. Alpers reports Consultancy: AstraZeneca, Mantra Bio, Novartis, Travere, and Variant Bio; and Research Funding: Sana. I. Bajema reports Employer: Pathan Laboratories Rotterdam and University Medical Center Groningen; Consultancy: Aurinia, Boehringer Ingelheim, CatBio, GSK, Hansa, Novartis, Otsuka, Toleranzia, Vera, and Vifor; Advisory or Leadership Role: C3 Glomerulopathy review board (Novartis, The Netherlands) and Glomerular Disease Council (Vifor Pharma); and Other Interests or Relationships: Director of Bajema Institute of Pathology, Past President of Renal Pathology Society, and Vice President of European Vasculitis Society (EUVAS). S.J. Barbour reports Consultancy: Achillion, Alexion, BeiGene, BioCryst, Eledon, HIBio, Inception Sciences, Novartis, Pfizer, Roche, Vera, and Visterra; Research Funding: Alexion, Novartis, and Roche; and Honoraria: Kirin. J.U. Becker reports Consultancy: Sanofi. H.T. Cook reports Consultancy: Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Novartis; and Research Funding: Alexion Pharmaceuticals. R. Coppo reports Consultancy: Amgen, Anylam, Argenx, Bayer, Calliditas, Chinook, Menarini, Novartis, Ostuka-Visterra, Purespring, Reata, Stadapharm; Honoraria: Amgen, Bayer, Chinook, Menarini, Novartis, Purespring, Stadapharm, and Travere; Patents or Royalties: UpToDate; Advisory or Leadership Role: Nephrology Dialysis Transplantation Editorial Board and
Figures

Similar articles
-
Clinical and histological comparison of IgA nephritis and renal IgA vasculitis.Nephrol Dial Transplant. 2024 Dec 20;40(1):182-192. doi: 10.1093/ndt/gfae143. Nephrol Dial Transplant. 2024. PMID: 38908911
-
IgA vasculitis nephritis: insights from kidney biopsies.Curr Opin Nephrol Hypertens. 2024 May 1;33(3):298-303. doi: 10.1097/MNH.0000000000000972. Epub 2024 Feb 19. Curr Opin Nephrol Hypertens. 2024. PMID: 38411035 Review.
-
Early clinical course of biopsy-proven IgA vasculitis nephritis.BMC Pediatr. 2022 Oct 4;22(1):570. doi: 10.1186/s12887-022-03611-9. BMC Pediatr. 2022. PMID: 36195856 Free PMC article.
-
Outcome of immunosuppression in children with IgA vasculitis-related nephritis.Nephrol Dial Transplant. 2024 Jul 31;39(8):1299-1309. doi: 10.1093/ndt/gfae009. Nephrol Dial Transplant. 2024. PMID: 38211969
-
Diagnosis and treatment of IgA nephropathy and IgA vasculitis nephritis in Chinese children.Pediatr Nephrol. 2023 Jun;38(6):1707-1715. doi: 10.1007/s00467-022-05798-6. Epub 2022 Nov 8. Pediatr Nephrol. 2023. PMID: 36348077 Free PMC article. Review.
Cited by
-
Urine complement-related proteins in IgA nephropathy and IgA vasculitis nephritis, possible biomarkers of disease activity.Clin Kidney J. 2024 Dec 3;18(1):sfae395. doi: 10.1093/ckj/sfae395. eCollection 2025 Jan. Clin Kidney J. 2024. PMID: 40008348 Free PMC article.
-
Navigating Adult-Onset IgA Vasculitis-Associated Nephritis.Life (Basel). 2024 Jul 25;14(8):930. doi: 10.3390/life14080930. Life (Basel). 2024. PMID: 39202674 Free PMC article. Review.
-
Systemic vasculitis involving the kidney: the nephropathologist's point of view.Pathologica. 2024 Apr;116(2):104-118. doi: 10.32074/1591-951X-990. Pathologica. 2024. PMID: 38767543 Free PMC article. Review.
-
Clinicopathological features and prognosis of IgA vasculitis nephritis with nephrotic-range proteinuria in children.Pediatr Nephrol. 2024 Nov;39(11):3241-3250. doi: 10.1007/s00467-024-06441-2. Epub 2024 Jul 9. Pediatr Nephrol. 2024. PMID: 38980322
-
APRIL: spring forward also for IgA vasculitis nephritis in children.Pediatr Nephrol. 2025 Jun 11. doi: 10.1007/s00467-025-06839-6. Online ahead of print. Pediatr Nephrol. 2025. PMID: 40498088 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

