Effector T Cells Promote Fibrosis in Corneal Transplantation Failure
- PMID: 38261311
- PMCID: PMC10810018
- DOI: 10.1167/iovs.65.1.40
Effector T Cells Promote Fibrosis in Corneal Transplantation Failure
Abstract
Purpose: To evaluate whether fibrosis contributes to corneal transplant failure and to determine whether effector CD4+ T cells, the key immune cells in corneal transplant rejection, play a direct role in fibrosis formation.
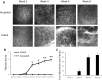
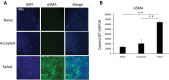
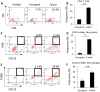
Methods: Allogeneic corneal transplantation was performed in mice. Graft opacity was evaluated by slit-lamp biomicroscopy, and fibrosis was assessed by in vivo confocal microscopy. Expression of alpha-smooth muscle actin (α-SMA) in both accepted and failed grafts was assessed by real-time PCR and immunohistochemistry. Frequencies of graft-infiltrating CD4+ T cells, neutrophils, and macrophages were assessed using flow cytometry. In vitro, MK/T-1 corneal fibroblasts were co-cultured with activated CD4+CD25- effector T cells isolated from corneal transplant recipient mice, and α-SMA expression was quantified by real-time PCR and ELISA. Neutralizing antibody was used to evaluate the role of interferon gamma (IFN-γ) in promoting α-SMA expression.
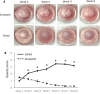
Results: The majority of failed grafts demonstrated clinical signs of fibrosis which became most evident at week 6 after corneal transplantation. Failed grafts showed higher expression of α-SMA as compared to accepted grafts. Flow cytometry analysis showed a significant increase in CD4+ T cells in failed grafts compared to accepted grafts. Co-culture of activated CD4+CD25- effector T cells with corneal fibroblasts led to an increase in α-SMA expression by fibroblasts. Inhibition of IFN-γ in culture significantly suppressed this increase in α-SMA expression as compared to immunoglobulin G control.
Conclusions: Fibrosis contributes to graft opacity in corneal transplant failure and is mediated at least in part by effector CD4+ T cells via IFN-γ.
Conflict of interest statement
Disclosure:
Figures

References
-
- Dana MR, Qian Y, Hamrah P. Twenty-five–year panorama of corneal immunology. Cornea. 2000; 19: 625–643. - PubMed
-
- Hamrah P. High-risk penetrating keratoplasty. Arch Soc Esp Oftalmol. 2005; 80: 5–7. - PubMed
-
- EBAA. 2014 Eye Banking Statistical Report. Washington, DC: Eye Bank Association of America; 2014.
-
- Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005; 140: 1112–1122. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

