Early Switch From Intravenous to Oral Antibiotics for Patients With Uncomplicated Gram-Negative Bacteremia
- PMID: 38261322
- PMCID: PMC10807296
- DOI: 10.1001/jamanetworkopen.2023.52314
Early Switch From Intravenous to Oral Antibiotics for Patients With Uncomplicated Gram-Negative Bacteremia
Abstract
Importance: Gram-negative bacteremia is a global health concern, and optimizing the transition from intravenous (IV) to oral antibiotics is a critical step in improving patient treatment and resource utilization.
Objective: To assess the association of switching to oral antibiotics within 4 days after initial blood culture with 90-day all-cause mortality compared with prolonged IV antibiotic treatment for patients with uncomplicated gram-negative bacteremia.
Design, setting, and participants: This cohort study conducted using the target trial emulation framework included observational data from adults with uncomplicated gram-negative bacteremia in 4 hospitals in Copenhagen, Denmark, from January 1, 2018, through December 31, 2021. The duration of follow-up was 90 days. Eligibility criteria included a blood culture positive for growth of gram-negative bacteria, clinical stability within 4 days of initial blood culture, an available susceptibility report on day 4, and initiation of appropriate empirical IV antibiotic treatment within 24 hours of blood culture.
Exposure: Switching to oral antibiotics within 4 days after initial blood culture compared with continuing IV antibiotic treatment for at least 5 days after initial blood culture.
Main outcomes and measures: The main outcome was 90-day all-cause mortality. Inverse probability of treatment weighting was applied to adjust for confounding. Intention-to-treat and per-protocol analyses were performed using pooled logistic regression to estimate absolute risk, risk difference (RD), and risk ratio (RR); 95% CIs were computed using bootstrapping.
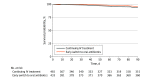
Results: A total of 914 individuals were included in the target trial emulation analysis (512 [56.0%] male; median age, 74.5 years [IQR, 63.3-83.2 years]); 433 (47.4%) transitioned early to oral antibiotic treatment, and 481 (52.6%) received prolonged IV treatment. Ninety-nine individuals (10.8%) died during follow-up. The proportion of individuals who died was higher in the group receiving prolonged IV treatment (69 [14.3%] vs 30 [6.9%]). In the intention-to-treat analysis, 90-day all-cause mortality risk was 9.1% (95% CI, 6.7%-11.6%) for the early-switch group and 11.7% (95% CI, 9.6%-13.8%) for the group receiving prolonged IV treatment; the RD was -2.5% (95% CI, -5.7% to 0.7%) and RR was 0.78 (95% CI, 0.60-1.10). In the per-protocol analysis, the RD was -0.1% (95% CI, -3.4% to 3.1%) and RR was 0.99 (95% CI, 0.70-1.40).
Conclusions and relevance: In this cohort study of uncomplicated gram-negative bacteremia, early transition to oral antibiotics within 4 days of initial blood culture was associated with 90-day all-cause mortality risk comparable to that of continuing IV antibiotic treatment and may be an effective alternative to prolonged IV treatment.
Conflict of interest statement
Figures
Comment in
-
"Real-World" Evidence, Target Trial Emulation, and Randomized Clinical Trials-Which Data Should Clinicians Rely on When Choosing Antibiotics?JAMA Netw Open. 2024 Jan 2;7(1):e2352250. doi: 10.1001/jamanetworkopen.2023.52250. JAMA Netw Open. 2024. PMID: 38261324 No abstract available.
References
-
- de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infection. 2013;19(9):860-868. - PubMed
-
- Kang CI, Kim SH, Park WB, et al. . Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760-766. doi:10.1128/AAC.49.2.760-766.2005 - DOI - PMC - PubMed
-
- Tamma PD, Conley AT, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group . Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. doi:10.1001/jamainternmed.2018.6226 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous