Intranasal Naloxone Repeat Dosing Strategies and Fentanyl Overdose: A Simulation-Based Randomized Clinical Trial
- PMID: 38261323
- PMCID: PMC10807299
- DOI: 10.1001/jamanetworkopen.2023.51839
Intranasal Naloxone Repeat Dosing Strategies and Fentanyl Overdose: A Simulation-Based Randomized Clinical Trial
Abstract
Importance: Questions have emerged as to whether standard intranasal naloxone dosing recommendations (ie, 1 dose with readministration every 2-3 minutes if needed) are adequate in the era of illicitly manufactured fentanyl and its derivatives (hereinafter, fentanyl).
Objective: To compare naloxone plasma concentrations between different intranasal naloxone repeat dosing strategies and to estimate their effect on fentanyl overdose.
Design, setting, and participants: This unblinded crossover randomized clinical trial was conducted with healthy participants in a clinical pharmacology unit (Spaulding Clinical Research, West Bend, Wisconsin) in March 2021. Inclusion criteria included age 18 to 55 years, nonsmoking status, and negative test results for the presence of alcohol or drugs of abuse. Data analysis was performed from October 2021 to May 2023.
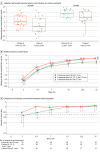
Intervention: Naloxone administered as 1 dose (4 mg/0.1 mL) at 0, 2.5, 5, and 7.5 minutes (test), 2 doses at 0 and 2.5 minutes (test), and 1 dose at 0 and 2.5 minutes (reference).
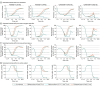
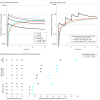
Main outcomes and measures: The primary outcome was the first prespecified time with higher naloxone plasma concentration. The secondary outcome was estimated brain hypoxia time following simulated fentanyl overdoses using a physiologic pharmacokinetic-pharmacodynamic model. Naloxone concentrations were compared using paired tests at 3 prespecified times across the 3 groups, and simulation results were summarized using descriptive statistics.
Results: This study included 21 participants, and 18 (86%) completed the trial. The median participant age was 34 years (IQR, 27-50 years), and slightly more than half of participants were men (11 [52%]). Compared with 1 naloxone dose at 0 and 2.5 minutes, 1 dose at 0, 2.5, 5, and 7.5 minutes significantly increased naloxone plasma concentration at 10 minutes (7.95 vs 4.42 ng/mL; geometric mean ratio, 1.95 [1-sided 97.8% CI, 1.28-∞]), whereas 2 doses at 0 and 2.5 minutes significantly increased the plasma concentration at 4.5 minutes (2.24 vs 1.23 ng/mL; geometric mean ratio, 1.98 [1-sided 97.8% CI, 1.03-∞]). No drug-related serious adverse events were reported. The median brain hypoxia time after a simulated fentanyl 2.97-mg intravenous bolus was 4.5 minutes (IQR, 2.1-∞ minutes) with 1 naloxone dose at 0 and 2.5 minutes, 4.5 minutes (IQR, 2.1-∞ minutes) with 1 naloxone dose at 0, 2.5, 5, and 7.5 minutes, and 3.7 minutes (IQR, 1.5-∞ minutes) with 2 naloxone doses at 0 and 2.5 minutes.
Conclusions and relevance: In this clinical trial with healthy participants, compared with 1 intranasal naloxone dose administered at 0 and 2.5 minutes, 1 dose at 0, 2.5, 5, and 7.5 minutes significantly increased naloxone plasma concentration at 10 minutes, whereas 2 doses at 0 and 2.5 minutes significantly increased naloxone plasma concentration at 4.5 minutes. Additional research is needed to determine optimal naloxone dosing in the community setting.
Trial registration: ClinicalTrials.gov Identifier: NCT04764630.
Conflict of interest statement
Figures

Similar articles
-
Naloxone Dosing After Opioid Overdose in the Era of Illicitly Manufactured Fentanyl.J Med Toxicol. 2020 Jan;16(1):41-48. doi: 10.1007/s13181-019-00735-w. Epub 2019 Aug 30. J Med Toxicol. 2020. PMID: 31471760 Free PMC article.
-
Effect of Intranasal vs Intramuscular Naloxone on Opioid Overdose: A Randomized Clinical Trial.JAMA Netw Open. 2019 Nov 1;2(11):e1914977. doi: 10.1001/jamanetworkopen.2019.14977. JAMA Netw Open. 2019. PMID: 31722024 Free PMC article. Clinical Trial.
-
Pharmacokinetics of concentrated naloxone nasal spray over first 30 minutes post-dosing: analysis of suitability for opioid overdose reversal.Addiction. 2017 Sep;112(9):1647-1652. doi: 10.1111/add.13849. Epub 2017 May 28. Addiction. 2017. PMID: 28430384 Clinical Trial.
-
Naloxone dosing in the era of ultra-potent opioid overdoses: a systematic review.CJEM. 2020 Mar;22(2):178-186. doi: 10.1017/cem.2019.471. CJEM. 2020. PMID: 31955714
-
Pharmacokinetic considerations for community-based dosing of nasal naloxone in opioid overdose in adults.Expert Opin Drug Metab Toxicol. 2022 Mar;18(3):203-217. doi: 10.1080/17425255.2022.2072728. Epub 2022 Jun 8. Expert Opin Drug Metab Toxicol. 2022. PMID: 35500297 Review.
Cited by
-
Comparison of intranasal naloxone and intranasal nalmefene in a translational model assessing the impact of synthetic opioid overdose on respiratory depression and cardiac arrest.Front Psychiatry. 2024 Jun 17;15:1399803. doi: 10.3389/fpsyt.2024.1399803. eCollection 2024. Front Psychiatry. 2024. PMID: 38952632 Free PMC article.
-
Oxygen fluctuations in the brain and periphery induced by intravenous fentanyl: effects of dose and drug experience.J Neurophysiol. 2024 Aug 1;132(2):322-334. doi: 10.1152/jn.00177.2024. Epub 2024 Jun 12. J Neurophysiol. 2024. PMID: 38863429 Free PMC article.
-
Synthetic opioids have disrupted conventional wisdom for treating opioid overdose.Drug Alcohol Depend Rep. 2024 Aug 13;12:100268. doi: 10.1016/j.dadr.2024.100268. eCollection 2024 Sep. Drug Alcohol Depend Rep. 2024. PMID: 39262668 Free PMC article.
-
A comparison of intramuscular (Zimhi) and intranasal naloxone (Narcan) in reversal of fentanyl-induced apnea: a randomized, crossover, open-label trial.Nat Commun. 2025 May 19;16(1):4659. doi: 10.1038/s41467-025-59932-7. Nat Commun. 2025. PMID: 40389500 Free PMC article. Clinical Trial.
-
Hypoxic effects of heroin and fentanyl and their basic physiological mechanisms.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L930-L948. doi: 10.1152/ajplung.00251.2024. Epub 2024 Oct 15. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39404797 Review.
References
-
- Ahmad FBAR, Cisewski JA, Rossen LM, Warner M, Sutton P. County-level provisional drug overdose death counts. Centers for Disease Control and Prevention National Center for Health Statistics. Accessed April 12, 2023. https://www.cdc.gov/nchs/nvss/vsrr/prov-county-drug-overdose.htm

