Examining B-cell dynamics and responsiveness in different inflammatory milieus using an agent-based model
- PMID: 38261584
- PMCID: PMC10805321
- DOI: 10.1371/journal.pcbi.1011776
Examining B-cell dynamics and responsiveness in different inflammatory milieus using an agent-based model
Abstract
Introduction: B-cells are essential components of the immune system that neutralize infectious agents through the generation of antigen-specific antibodies and through the phagocytic functions of naïve and memory B-cells. However, the B-cell response can become compromised by a variety of conditions that alter the overall inflammatory milieu, be that due to substantial, acute insults as seen in sepsis, or due to those that produce low-level, smoldering background inflammation such as diabetes, obesity, or advanced age. This B-cell dysfunction, mediated by the inflammatory cytokines Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α), increases the susceptibility of late-stage sepsis patients to nosocomial infections and increases the incidence or severity of recurrent infections, such as SARS-CoV-2, in those with chronic conditions. We propose that modeling B-cell dynamics can aid the investigation of their responses to different levels and patterns of systemic inflammation.
Methods: The B-cell Immunity Agent-based Model (BCIABM) was developed by integrating knowledge regarding naïve B-cells, short-lived plasma cells, long-lived plasma cells, memory B-cells, and regulatory B-cells, along with their various differentiation pathways and cytokines/mediators. The BCIABM was calibrated to reflect physiologic behaviors in response to: 1) mild antigen stimuli expected to result in immune sensitization through the generation of effective immune memory, and 2) severe antigen challenges representing the acute substantial inflammation seen during sepsis, previously documented in studies on B-cell behavior in septic patients. Once calibrated, the BCIABM was used to simulate the B-cell response to repeat antigen stimuli during states of low, chronic background inflammation, implemented as low background levels of IL-6 and TNF-α often seen in patients with conditions such as diabetes, obesity, or advanced age. The levels of immune responsiveness were evaluated and validated by comparing to a Veteran's Administration (VA) patient cohort with COVID-19 infection known to have a higher incidence of such comorbidities.
Results: The BCIABM was successfully able to reproduce the expected appropriate development of immune memory to mild antigen exposure, as well as the immunoparalysis seen in septic patients. Simulation experiments then revealed significantly decreased B-cell responsiveness as levels of background chronic inflammation increased, reproducing the different COVID-19 infection data seen in a VA population.
Conclusion: The BCIABM proved useful in dynamically representing known mechanisms of B-cell function and reproduced immune memory responses across a range of different antigen exposures and inflammatory statuses. These results elucidate previous studies demonstrating a similar negative correlation between the B-cell response and background inflammation by positing an established and conserved mechanism that explains B-cell dysfunction across a wide range of phenotypic presentations.
Copyright: © 2024 Shin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
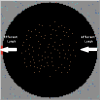
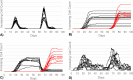


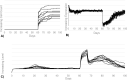
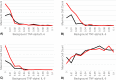
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

