Restoring immune tolerance in pemphigus vulgaris
- PMID: 38261616
- PMCID: PMC10835025
- DOI: 10.1073/pnas.2317762121
Restoring immune tolerance in pemphigus vulgaris
Abstract
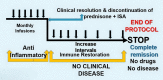
Intravenous immunoglobulin (IVIg), a preparation of polyclonal serum IgG pooled from numerous blood donors, has been used for nearly three decades and is proving to be an efficient treatment for many autoimmune blistering diseases, including pemphigus vulgaris (PV). Despite its widespread use and therapeutic success, its mechanisms of action are not completely understood. Some of its anti-inflammatory and immunomodulatory actions have been studied. In this study, the authors present a twenty-year follow-up of 21 patients with clinical and immunopathological confirmed PV, treated with IVIg as monotherapy, according to an established published protocol. IVIg therapy produced long-term sustained, clinical, serological, and immunopathological remission. For 20 y, these patients received no drugs and experienced no disease. This observation suggests that there was the establishment of immune balance or restoration of immune regulation in these PV patients. Twelve (57%) patients experienced no relapse during follow-up. Six (29%) patients experienced a relapse due to acute stress or post-coronavirus infection and/or vaccination. Reinstitution of IVIg resulted in prompt sustained recovery. Three (14.2%) patients, in clinical and serological remission, died due to unrelated causes. No severe adverse effects from IVIg were documented in all 21 patients. The simultaneous or sequential anti-inflammatory and immunomodulatory effects of IVIg may have influenced the long-term clinical remission observed. This study provides a human prototype to examine the pathophysiology of autoimmunity and a model to study immune regulation and mechanisms that can facilitate restoring immune tolerance.
Keywords: immune tolerance; intravenous immunoglobulin; long-term remission; pemphigus vulgaris; twenty-year follow-up.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Ruocco E., Baroni A., Wolf R., Ruocco V., Life-threatening bullous dermatoses: Pemphigus vulgaris. Clin. Dermatol. 23, 223–226 (2005). - PubMed
-
- Kridin K., Pemphigus group: Overview, epidemiology, mortality, and comorbidities. Immunol. Res. 66, 255–270 (2018). - PubMed
-
- Reznick L., Lever W. F., Frazier C. N., Treatment of pemphigus with ACTH, cortisone and prednisone; results obtained in twenty-five cases over a period of five years. N Engl. J. Med. 255, 305–315 (1956). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

