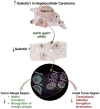
Targeting stroma and tumor, silencing galectin 1 treats orthotopic mouse hepatocellular carcinoma
- PMID: 38261802
- PMCID: PMC10793093
- DOI: 10.1016/j.apsb.2023.10.010
Targeting stroma and tumor, silencing galectin 1 treats orthotopic mouse hepatocellular carcinoma
Abstract
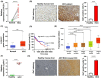
This study examines inhibiting galectin 1 (Gal1) as a treatment option for hepatocellular carcinoma (HCC). Gal1 has immunosuppressive and cancer-promoting roles. Our data showed that Gal1 was highly expressed in human and mouse HCC. The levels of Gal1 positively correlated with the stages of human HCC and negatively with survival. The roles of Gal1 in HCC were studied using overexpression (OE) or silencing using Igals1 siRNA delivered by AAV9. Prior to HCC initiation induced by RAS and AKT mutations, lgals1-OE and silencing had opposite impacts on tumor load. The treatment effect of lgals1 siRNA was further demonstrated by intersecting HCC at different time points when the tumor load had already reached 9% or even 42% of the body weight. Comparing spatial transcriptomic profiles of Gal1 silenced and OE HCC, inhibiting matrix formation and recognition of foreign antigen in CD45+ cell-enriched areas located at tumor-margin likely contributed to the anti-HCC effects of Gal1 silencing. Within the tumors, silencing Gal1 inhibited translational initiation, elongation, and termination. Furthermore, Gal1 silencing increased immune cells as well as expanded cytotoxic T cells within the tumor, and the anti-HCC effect of lgals1 siRNA was CD8-dependent. Overall, Gal1 silencing has a promising potential for HCC treatment.
Keywords: Carbohydrate-binding lectin; Extracellular matrix; Galectin 1; Hepatocellular carcinoma; Liver; Spatial transcriptomics; Translation; Tumor-margin.
© 2024 The Authors.
Conflict of interest statement
The authors declare no potential competing interests.
Figures




References
-
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. - PubMed
-
- Perillo N.L., Pace K.E., Seilhamer J.J., Baum L.G. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

