A multidimensional platform of patient-derived tumors identifies drug susceptibilities for clinical lenvatinib resistance
- PMID: 38261805
- PMCID: PMC10793100
- DOI: 10.1016/j.apsb.2023.09.015
A multidimensional platform of patient-derived tumors identifies drug susceptibilities for clinical lenvatinib resistance
Abstract
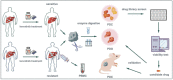
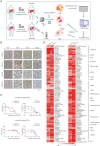
Lenvatinib, a second-generation multi-receptor tyrosine kinase inhibitor approved by the FDA for first-line treatment of advanced liver cancer, facing limitations due to drug resistance. Here, we applied a multidimensional, high-throughput screening platform comprising patient-derived resistant liver tumor cells (PDCs), organoids (PDOs), and xenografts (PDXs) to identify drug susceptibilities for conquering lenvatinib resistance in clinically relevant settings. Expansion and passaging of PDCs and PDOs from resistant patient liver tumors retained functional fidelity to lenvatinib treatment, expediting drug repurposing screens. Pharmacological screening identified romidepsin, YM155, apitolisib, NVP-TAE684 and dasatinib as potential antitumor agents in lenvatinib-resistant PDC and PDO models. Notably, romidepsin treatment enhanced antitumor response in syngeneic mouse models by triggering immunogenic tumor cell death and blocking the EGFR signaling pathway. A combination of romidepsin and immunotherapy achieved robust and synergistic antitumor effects against lenvatinib resistance in humanized immunocompetent PDX models. Collectively, our findings suggest that patient-derived liver cancer models effectively recapitulate lenvatinib resistance observed in clinical settings and expedite drug discovery for advanced liver cancer, providing a feasible multidimensional platform for personalized medicine.
Keywords: Drug discovery; Drug resistance; EGFR; High-throughput screening; Lenvatinib; Liver cancer; Patient-derived model; Romidepsin.
© 2024 The Authors.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

