PRESTO: A Phase III, Open-Label Study of Intensification of Androgen Blockade in Patients With High-Risk Biochemically Relapsed Castration-Sensitive Prostate Cancer (AFT-19)
- PMID: 38261983
- PMCID: PMC11637124
- DOI: 10.1200/JCO.23.01157
PRESTO: A Phase III, Open-Label Study of Intensification of Androgen Blockade in Patients With High-Risk Biochemically Relapsed Castration-Sensitive Prostate Cancer (AFT-19)
Abstract
Purpose: Patients with biochemically recurrent prostate cancer (BRPC) after radical prostatectomy and a short PSA doubling time are at risk for distant metastases. Apalutamide, an androgen receptor antagonist, and abiraterone acetate plus prednisone (AAP) prolong survival in the metastatic setting. We evaluated whether intensification of androgen-deprivation therapy (ADT) improves outcomes in BRPC.
Patients and methods: PRESTO is a randomized phase III, open-label trial in patients with BRPC and PSA doubling time ≤9 months (ClinicalTrials.gov identifier: NCT03009981). Patients were randomly assigned 1:1:1 to receive a finite 52-week treatment course with ADT control, ADT + apalutamide, or ADT + apalutamide + AAP. The primary end point was PSA progression-free survival (PSA-PFS), defined as serum PSA >0.2 ng/mL after treatment completion.
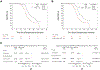
Results: Five hundred three patients were enrolled. The median PSA was 1.8 ng/mL (IQR, 1.0-3.6). At the first planned interim analysis, both experimental arms significantly prolonged PSA-PFS compared with the control arm (median, 24.9 months for ADT + apalutamide v 20.3 months for ADT; hazard ratio [HR], 0.52 [95% CI, 0.35 to 0.77]; P = .00047; median, 26.0 months for ADT + apalutamide + AAP v 20.0 months for ADT; HR, 0.48 [95% CI, 0.32 to 0.71]; P = .00008). Median time to testosterone recovery did not differ across treatment arms. The most common grade ≥3 adverse event was hypertension (7.5%, 7.4%, and 18% in ADT, ADT + apalutamide, and ADT + apalutamide + AAP arms, respectively).
Conclusion: Intensified AR blockade for a finite duration prolongs PSA-PFS with a manageable safety profile, without adversely affecting time to testosterone recovery. The addition of apalutamide to ADT should be considered in patients with high-risk BRPC.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rahul Aggarwal
Joel Picus
Mary-Ellen Taplin
Tanya Dorff
Leonard Appleman
Akash Patnaik
Alan Bryce
James Mohler
Arpit Rao
Scott Tagawa
Alan Tan
Susan Halabi
Patrick O'Brien
Ronald Chen
Charles Ryan
Scott E. Eggener
Michael J. Morris
No other potential conflicts of interest were reported.
Figures


Comment in
-
ADT intensification to treat biochemically recurrent prostate cancer.Nat Rev Urol. 2024 Mar;21(3):126. doi: 10.1038/s41585-024-00862-2. Nat Rev Urol. 2024. PMID: 38355924 No abstract available.
References
-
- Siegel RL, Miller KD, Wagle NS, et al.: Cancer statistics, 2023. CA Cancer J Clin 73:17–48, 2023 - PubMed
-
- Culp MB, Soerjomataram I, Efstathiou JA, et al.: Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 77:38–52, 2020 - PubMed
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program, http://seer.cancer.gov/statfacts/html/prost.html
-
- Caire AA, Sun L, Ode O, et al.: Delayed prostate-specific antigen recurrence after radical prostatectomy: How to identify and what are their clinical outcomes? Urology 74:643–647, 2009 - PubMed
-
- Pound CR, Partin AW, Eisenberger MA, et al.: Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281:1591–1597, 1999 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

