Cell surface RNAs control neutrophil recruitment
- PMID: 38262409
- PMCID: PMC10922858
- DOI: 10.1016/j.cell.2023.12.033
Cell surface RNAs control neutrophil recruitment
Abstract
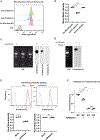
RNAs localizing to the outer cell surface have been recently identified in mammalian cells, including RNAs with glycan modifications known as glycoRNAs. However, the functional significance of cell surface RNAs and their production are poorly known. We report that cell surface RNAs are critical for neutrophil recruitment and that the mammalian homologs of the sid-1 RNA transporter are required for glycoRNA expression. Cell surface RNAs can be readily detected in murine neutrophils, the elimination of which substantially impairs neutrophil recruitment to inflammatory sites in vivo and reduces neutrophils' adhesion to and migration through endothelial cells. Neutrophil glycoRNAs are predominantly on cell surface, important for neutrophil-endothelial interactions, and can be recognized by P-selectin (Selp). Knockdown of the murine Sidt genes abolishes neutrophil glycoRNAs and functionally mimics the loss of cell surface RNAs. Our data demonstrate the biological importance of cell surface glycoRNAs and highlight a noncanonical dimension of RNA-mediated cellular functions.
Keywords: 45S rRNA; E-selectin; Sidt1; Sidt2; peritonitis; snoRNA; tRNA.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

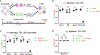



References
-
- Wang GG, Calvo KR, Pasillas MP, Sykes DB, Häcker H, and Kamps MP (2006). Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293. - PubMed
-
- Orosz A, Walzog B, and Mócsai A (2021). In vivo functions of mouse neutrophils derived from HoxB8-transduced conditionally immortalized myeloid progenitors. J. Immunol 206, 432–445. - PubMed
-
- McDonald JU, Cortini A, Rosas M, Fossati-Jimack L, Ling GS, Lewis KJ, Dewitt S, Liddiard K, Brown GD, Jones SA, et al. (2011). In vivo functional analysis and genetic modification of in vitro -derived mouse neutrophils. FASEB J 25, 1972–1982. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

