Long-Term Survival in Patients With Relapsed/Refractory Advanced Renal Cell Carcinoma Treated With Tivozanib: Analysis of the Phase III TIVO-3 Trial
- PMID: 38262444
- PMCID: PMC10911910
- DOI: 10.1093/oncolo/oyad348
Long-Term Survival in Patients With Relapsed/Refractory Advanced Renal Cell Carcinoma Treated With Tivozanib: Analysis of the Phase III TIVO-3 Trial
Abstract
Background: Tivozanib is an oral vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) with efficacy in advanced renal cell carcinoma (RCC). Long-term exploratory analyses from the TIVO-3 trial in relapsed/refractory (R/R) RCC including patients (26%) with prior immuno-oncology (IO) therapy are reported.
Methods: Patients with R/R advanced RCC that progressed with 2 or 3 prior systemic therapies (≥1 VEGFR TKI) were randomized to tivozanib 1.5 mg QD or sorafenib 400 mg BID, stratified by IMDC risk and previous therapy. Safety, investigator-assessed long-term progression-free survival (LT-PFS), and serial overall survival (OS) were assessed.
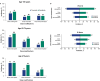
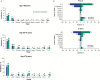
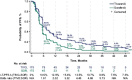
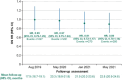
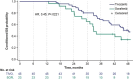
Results: Mean time on treatment was 11.0 months with tivozanib (n = 175) and 6.3 months with sorafenib (n = 175). Fewer grade ≥3 treatment-related adverse events occurred with tivozanib (46%) than sorafenib (55%). Dose modification rates were lower with tivozanib than sorafenib across age/prior IO subgroups; prior IO therapy did not impact dose reductions or discontinuations in either arm. Landmark LT-PFS rates were higher with tivozanib (3 years: 12.3% vs 2.4%; 4 years: 7.6% vs 0%). After 22.8 months mean follow-up, the OS HR was 0.89 (95% CI, 0.70-1.14); when conditioned on 12-month landmark PFS, tivozanib showed significant OS improvement over sorafenib (HR, 0.45; 95% CI, 0.22-0.91; 2-sided P = .0221).
Conclusions: Tivozanib demonstrated a consistent safety profile and long-term survival benefit in patients with R/R advanced RCC who were alive and progression free at 12 months. These post hoc exploratory analyses of LT-PFS and conditional OS support a clinically meaningful improvement with tivozanib versus sorafenib in this advanced RCC population.
Keywords: carcinoma; long-term progression free survival; relapsed kidney cancer; renal cell; tivozanib; vascular endothelial growth factor.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Kathryn E. Beckermann: Consulting or advisory role: Alpine Bioscience, Aravive, AVEO, AstraZeneca, Merck, Exelixis, Bristol Myers Squibb, Sanofi, Seagen; Research funding to the institution: Lung Cancer Foundation of America-International Association for the Study of Lung Cancer-Bristol Myers Squibb, Arsenal Bioscience, Aravive, Pionyr. Aviva G. Asnis-Alibozek: Consulting or advisory role: AVEO. Michael B. Atkins: Consulting or advisory role: Bristol Myers Squibb, Merck, Novartis, Eisai, AVEO, Pfizer, Werewolf, Fathom, Pyxis Oncology, Elpis, X4Pharma, ValoHealth, ScholarRock, Surface, Takeda, Roche, SAB Bio, Exelixis, Iovance, Idera, Agenus, Asher Bio, AstraZeneca, Seagen, Sanofi, OncoRena, Pliant Therapeutics, GSK, Atreca, Simcha; Stock options: Werewolf, Pyxis Oncology, Elpis. Bernard Escudier: Honoraria: Pfizer, Bristol Myers Squibb, Ipsen, Oncorena; Consulting or advisory role: Pfizer, Bristol Myers Squibb, Ipsen, AVEO, Oncorena; Research funding: Bristol Myers Squibb France; Travel, accommodations, expenses: Bristol Myers Squibb, Ipsen, MSD. Thomas E. Hutson: Honoraria: Pfizer, Bayer, GSK, Merck, AVEO, Eisai, Novartis, Exelixis; Consulting or advisory role: Pfizer, Bayer, GSK, Merck, AVEO, Eisai, Novartis, Exelixis; Research funding: Pfizer, Bayer, GSK, Merck, AVEO, Eisai, Novartis, Exelixis; Vijay Kasturi: Employment: AVEO. David F. McDermott: Honoraria: Bristol Myers Squibb, Pfizer, Merck, Eisai Inc, Xilio, AVEO, Genentech, Cullinan, Exelixis; Research funding: Bristol Myers Squibb, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, Alkermes, Inc. Sumanta K. Pal: Travel, accommodations, expenses: Ipsen, CRISPR Therapeutics. Camillo Porta: Consulting or advisory role: Angelini Pharma, AstraZeneca, Biorek, Bristol Myers Squibb, Eisai, Ipsen, Medendi, MSD; Speakers bureau: Angelini Pharma, Bristol Myers Squibb, Eisai, Ipsen, MSD. Brian I. Rini: Consulting or advisory role: Bristol Myers Squibb, Pfizer, GNE/Roche, AVEO, Synthorx, Merck, Corvus, Surface Oncology, Aravive, Alkermes, Arrowhead, Eisai, Nikang Therapeutics, EUSA, Athenex, Debiopharm, HiberCell; Research funding to the institution: AVEO, Arcus, Merck, Dragonfly Therapeutics, HiberCell, Incyte, Stata Oncology, ADC Therapeutics, Dracen Pharmaceuticals, Janssen, Adela, AstraZeneca, Pionyr, Tempus, VasGene Therapeutics, Gilead, POINT Biopharma, Bristol Myers Squibb, Pfizer, Daiichi Sankyo, Genentech, Arrowhead Pharmaceuticals, Exelixis, Surface Oncology, Aravive. Elena Verzoni: Consulting or advisory role: MSD, AstraZeneca, Ipsen, Bristol Myers Squibb, Janssen; Speakers bureau: MSD, AstraZeneca, Ipsen, Janssen, Astellas, Pfizer, Bristol Myers Squibb.
Figures
Similar articles
-
Updated overall survival in patients with prior checkpoint inhibitor therapy in the phase III TIVO-3 study.Oncologist. 2025 Feb 6;30(2):oyae369. doi: 10.1093/oncolo/oyae369. Oncologist. 2025. PMID: 39912344 Free PMC article. Clinical Trial.
-
Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial.J Clin Oncol. 2013 Oct 20;31(30):3791-9. doi: 10.1200/JCO.2012.47.4940. Epub 2013 Sep 9. J Clin Oncol. 2013. PMID: 24019545 Free PMC article. Clinical Trial.
-
The role of tivozanib in advanced renal cell carcinoma therapy.Expert Rev Anticancer Ther. 2018 Nov;18(11):1113-1124. doi: 10.1080/14737140.2018.1508348. Epub 2018 Aug 21. Expert Rev Anticancer Ther. 2018. PMID: 30084668 Review.
-
FDA Approval Summary: Tivozanib for Relapsed or Refractory Renal Cell Carcinoma.Clin Cancer Res. 2022 Feb 1;28(3):441-445. doi: 10.1158/1078-0432.CCR-21-2334. Epub 2021 Aug 20. Clin Cancer Res. 2022. PMID: 34417198 Free PMC article.
-
Tivozanib for the treatment of renal cell carcinoma: results and implications of the TIVO-1 trial.Future Oncol. 2014 Aug;10(11):1819-26. doi: 10.2217/fon.14.120. Future Oncol. 2014. PMID: 25325825 Review.
Cited by
-
A real-world disproportionality analysis of Tivozanib data mining of the public version of FDA adverse event reporting system.Front Pharmacol. 2024 Jun 13;15:1408135. doi: 10.3389/fphar.2024.1408135. eCollection 2024. Front Pharmacol. 2024. PMID: 38939844 Free PMC article.
-
Updated overall survival in patients with prior checkpoint inhibitor therapy in the phase III TIVO-3 study.Oncologist. 2025 Feb 6;30(2):oyae369. doi: 10.1093/oncolo/oyae369. Oncologist. 2025. PMID: 39912344 Free PMC article. Clinical Trial.
-
Establishment and characterization of NCC-SFT1-C1: a novel patient-derived cell line of solitary fibrous tumor.Hum Cell. 2025 Feb 4;38(2):49. doi: 10.1007/s13577-025-01175-1. Hum Cell. 2025. PMID: 39904835
-
Real-World Treatment Patterns and Clinical Outcomes Among Patients with Metastatic Renal Cell Carcinoma Post-Immune-Oncology and Vascular Endothelial Growth Factor Receptor Targeted Therapies.Cancers (Basel). 2025 Apr 25;17(9):1434. doi: 10.3390/cancers17091434. Cancers (Basel). 2025. PMID: 40361361 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

