Plectin plays a role in the migration and volume regulation of astrocytes: a potential biomarker of glioblastoma
- PMID: 38263015
- PMCID: PMC10807171
- DOI: 10.1186/s12929-024-01002-z
Plectin plays a role in the migration and volume regulation of astrocytes: a potential biomarker of glioblastoma
Abstract
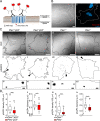
Background: The expression of aquaporin 4 (AQP4) and intermediate filament (IF) proteins is altered in malignant glioblastoma (GBM), yet the expression of the major IF-based cytolinker, plectin (PLEC), and its contribution to GBM migration and invasiveness, are unknown. Here, we assessed the contribution of plectin in affecting the distribution of plasmalemmal AQP4 aggregates, migratory properties, and regulation of cell volume in astrocytes.
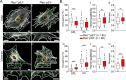
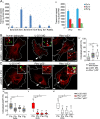
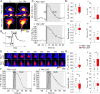
Methods: In human GBM, the expression of glial fibrillary acidic protein (GFAP), AQP4 and PLEC transcripts was analyzed using publicly available datasets, and the colocalization of PLEC with AQP4 and with GFAP was determined by immunohistochemistry. We performed experiments on wild-type and plectin-deficient primary and immortalized mouse astrocytes, human astrocytes and permanent cell lines (U-251 MG and T98G) derived from a human malignant GBM. The expression of plectin isoforms in mouse astrocytes was assessed by quantitative real-time PCR. Transfection, immunolabeling and confocal microscopy were used to assess plectin-induced alterations in the distribution of the cytoskeleton, the influence of plectin and its isoforms on the abundance and size of plasmalemmal AQP4 aggregates, and the presence of plectin at the plasma membrane. The release of plectin from cells was measured by ELISA. The migration and dynamics of cell volume regulation of immortalized astrocytes were assessed by the wound-healing assay and calcein labeling, respectively.
Results: A positive correlation was found between plectin and AQP4 at the level of gene expression and protein localization in tumorous brain samples. Deficiency of plectin led to a decrease in the abundance and size of plasmalemmal AQP4 aggregates and altered distribution and bundling of the cytoskeleton. Astrocytes predominantly expressed P1c, P1e, and P1g plectin isoforms. The predominant plectin isoform associated with plasmalemmal AQP4 aggregates was P1c, which also affected the mobility of astrocytes most prominently. In the absence of plectin, the collective migration of astrocytes was impaired and the dynamics of cytoplasmic volume changes in peripheral cell regions decreased. Plectin's abundance on the plasma membrane surface and its release from cells were increased in the GBM cell lines.
Conclusions: Plectin affects cellular properties that contribute to the pathology of GBM. The observed increase in both cell surface and released plectin levels represents a potential biomarker and therapeutic target in the diagnostics and treatment of GBMs.
Keywords: Aquaporin 4; Astrocyte; Cell migration; Cell volume; Cytoskeleton; Edema; Glioblastoma; Intermediate filaments; Plectin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Evidence of Aquaporin 4 Regulation by Thyroid Hormone During Mouse Brain Development and in Cultured Human Glioblastoma Multiforme Cells.Front Neurosci. 2019 Apr 4;13:317. doi: 10.3389/fnins.2019.00317. eCollection 2019. Front Neurosci. 2019. PMID: 31019448 Free PMC article.
-
Intermediate filament-associated cytolinker plectin 1c destabilizes microtubules in keratinocytes.Mol Biol Cell. 2013 Mar;24(6):768-84. doi: 10.1091/mbc.E12-06-0488. Epub 2013 Jan 30. Mol Biol Cell. 2013. PMID: 23363598 Free PMC article.
-
Aquaporin 4-dependent expression of glial fibrillary acidic protein and tenascin-C in activated astrocytes in stab wound mouse brain and in primary culture.J Neurosci Res. 2015 Jan;93(1):121-9. doi: 10.1002/jnr.23467. Epub 2014 Sep 1. J Neurosci Res. 2015. PMID: 25174305
-
Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes.Cells. 2020 Dec 7;9(12):2622. doi: 10.3390/cells9122622. Cells. 2020. PMID: 33297299 Free PMC article. Review.
-
AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology.Neuroscience. 2009 Jul 7;161(3):764-72. doi: 10.1016/j.neuroscience.2009.03.069. Epub 2009 Apr 5. Neuroscience. 2009. PMID: 19345723 Review.
Cited by
-
Plectin: Dual Participation in Tumor Progression.Biomolecules. 2024 Aug 24;14(9):1050. doi: 10.3390/biom14091050. Biomolecules. 2024. PMID: 39334817 Free PMC article. Review.
-
High matrix stiffness accelerates migration of hepatocellular carcinoma cells through the integrin β1-Plectin-F-actin axis.BMC Biol. 2025 Jan 9;23(1):8. doi: 10.1186/s12915-025-02113-1. BMC Biol. 2025. PMID: 39789506 Free PMC article.
-
Rap2B drives tumorigenesis and progression of colorectal cancer through intestinal cytoskeleton remodeling.Cell Death Dis. 2025 Apr 13;16(1):290. doi: 10.1038/s41419-025-07627-8. Cell Death Dis. 2025. PMID: 40223002 Free PMC article.
-
Single-cell sequencing elucidates the mechanism of NUSAP1 in glioma and its diagnostic and prognostic significance.Front Immunol. 2025 Feb 5;16:1512867. doi: 10.3389/fimmu.2025.1512867. eCollection 2025. Front Immunol. 2025. PMID: 39975552 Free PMC article.
-
Homozygosity for a Rare Plec Variant Suggests a Contributory Role in Congenital Insensitivity to Pain.Int J Mol Sci. 2024 Jun 8;25(12):6358. doi: 10.3390/ijms25126358. Int J Mol Sci. 2024. PMID: 38928066 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

