In vivo assembly of epitope-coated biopolymer particles that induce anti-tumor responses
- PMID: 38263169
- PMCID: PMC10805745
- DOI: 10.1038/s41541-023-00787-8
In vivo assembly of epitope-coated biopolymer particles that induce anti-tumor responses
Abstract
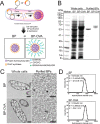
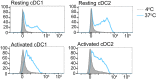
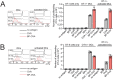
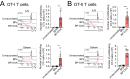
There is an unmet need for antigen delivery systems that elicit efficient T cell priming to prevent infectious diseases or for treatment of cancers. Here, we explored the immunogenic potential of biologically assembled biopolymer particles (BPs) that have been bioengineered to display the antigenic MHC I and MHC II epitopes of model antigen ovalbumin (OVA). Purified dendritic cells (DCs) captured BP-OVA and presented the associated antigenic epitopes to CD4+ T cells and CD8+ T cells. Vaccination with BP-OVA in the absence of adjuvant elicited antigen presentation to OVA-specific CD8+ and CD4+ T cells and cross-primed effective cytotoxic T lymphocyte (CTL) killers. BP-OVA induction of CTL killing did not require CD4+ T cell help, with active CTLs generated in BP-OVA vaccinated I-Ab-/- and CD40-/- mice. In contrast, IL-15 and type I IFN were required, with abrogated CTL activity in vaccinated IL-15-/- and IFNAR1-/- mice. cDC1 and/or CD103+ DCs were not essential for BP-OVA specific CTL with immunization eliciting responses in Batf3-/- mice. Poly I:C, but not LPS or CpG, co-administered as an adjuvant with BP-OVA boosted CTL responses. Finally, vaccination with BP-OVA protected against B16-OVA melanoma and Eμ-myc-GFP-OVA lymphoma inoculation. In summary, we have demonstrated that epitope-displaying BPs represent an antigen delivery platform exhibiting a unique mechanism to effectively engage T cell immune responses.
© 2024. The Author(s).
Conflict of interest statement
B.H.A.R. is inventor of the BP technology and co-founder and shareholder of PolyBatics Ltd. The remaining authors declare no competing interests.
Figures



Similar articles
-
Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC.J Immunol. 1998 Dec 15;161(12):6532-6. J Immunol. 1998. PMID: 9862678
-
Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses.PLoS One. 2014 Jun 9;9(6):e99396. doi: 10.1371/journal.pone.0099396. eCollection 2014. PLoS One. 2014. PMID: 24911024 Free PMC article.
-
Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors.Cancer Sci. 2008 May;99(5):1008-15. doi: 10.1111/j.1349-7006.2008.00788.x. Epub 2008 Mar 12. Cancer Sci. 2008. PMID: 18341654 Free PMC article.
-
Intradermal injections of polyarginine-containing immunogenic antigens preferentially elicit Tc1 and Th1 activation and antitumour immunity.Br J Dermatol. 2010 Jan;162(1):29-41. doi: 10.1111/j.1365-2133.2009.09490.x. Epub 2009 Oct 26. Br J Dermatol. 2010. PMID: 19863514
-
A novel T cell-based vaccine capable of stimulating long-term functional CTL memory against B16 melanoma via CD40L signaling.Cell Mol Immunol. 2013 Jan;10(1):72-7. doi: 10.1038/cmi.2012.37. Epub 2012 Oct 8. Cell Mol Immunol. 2013. PMID: 23042534 Free PMC article.
Cited by
-
A KIF20A-based thermosensitive hydrogel vaccine effectively potentiates immune checkpoint blockade therapy for hepatocellular carcinoma.NPJ Vaccines. 2025 Jan 3;10(1):1. doi: 10.1038/s41541-024-01060-2. NPJ Vaccines. 2025. PMID: 39753573 Free PMC article.
References
-
- Hay, I. D., Hooks, D. O. & Rehm, B. H. A. Use of bacterial polyhydroxyalkanoates in protein display use of bacterial polyhydroxyalkanoates in protein display technologies. Hydrocarbon Lipid Microbiol. Protoc.10.1007/8623 (2014).
Grants and funding
- 1161101/Department of Health | National Health and Medical Research Council (NHMRC)
- 1129672/Department of Health | National Health and Medical Research Council (NHMRC)
- 180100844/Department of Education and Training | Australian Research Council (ARC)
- 160101373/Department of Education and Training | Australian Research Council (ARC)
- 180100521/Department of Education and Training | Australian Research Council (ARC)
- 190101242/Department of Education and Training | Australian Research Council (ARC)
- DP200100874/Department of Education and Training | Australian Research Council (ARC)
- LE20010014/Department of Education and Training | Australian Research Council (ARC)
- DP200100874/Department of Education and Training | Australian Research Council (ARC)
- LE20010014/Department of Education and Training | Australian Research Council (ARC)
LinkOut - more resources
Full Text Sources
Research Materials

