In vivo identification of Drosophila rhodopsin interaction partners by biotin proximity labeling
- PMID: 38263196
- PMCID: PMC10805788
- DOI: 10.1038/s41598-024-52041-3
In vivo identification of Drosophila rhodopsin interaction partners by biotin proximity labeling
Abstract
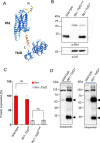
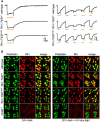
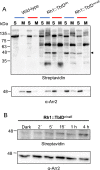
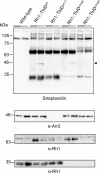
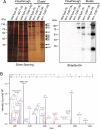
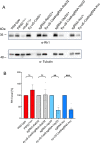
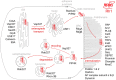
Proteins exert their function through protein-protein interactions. In Drosophila, G protein-coupled receptors like rhodopsin (Rh1) interact with a G protein to activate visual signal transduction and with arrestins to terminate activation. Also, membrane proteins like Rh1 engage in protein-protein interactions during folding within the endoplasmic reticulum, during their vesicular transport and upon removal from the cell surface and degradation. Here, we expressed a Rh1-TurboID fusion protein (Rh1::TbID) in Drosophila photoreceptors to identify in vivo Rh1 interaction partners by biotin proximity labeling. We show that Rh1::TbID forms a functional rhodopsin that mediates biotinylation of arrestin 2 in conditions where arrestin 2 interacts with rhodopsin. We also observed biotinylation of Rh1::TbID and native Rh1 as well as of most visual signal transduction proteins. These findings indicate that the signaling components in the rhabdomere approach rhodopsin closely, within a range of ca. 10 nm. Furthermore, we have detected proteins engaged in the maturation of rhodopsin and elements responsible for the trafficking of membrane proteins, resembling potential interaction partners of Rh1. Among these are chaperons of the endoplasmic reticulum, proteins involved in Clathrin-mediated endocytosis as well as previously unnoticed contributors to rhodopsin transportation, such as Rab32, Vap33, or PIP82.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Differential activation of rhodopsin triggers distinct endocytic trafficking and recycling in vivo via differential phosphorylation.PLoS One. 2024 Jun 7;19(6):e0303882. doi: 10.1371/journal.pone.0303882. eCollection 2024. PLoS One. 2024. PMID: 38848405 Free PMC article.
-
Distinct roles of arrestin 1 protein in photoreceptors during Drosophila development.J Biol Chem. 2014 Jun 27;289(26):18526-34. doi: 10.1074/jbc.M114.571224. Epub 2014 May 16. J Biol Chem. 2014. PMID: 24838243 Free PMC article.
-
Role of rhodopsin and arrestin phosphorylation in retinal degeneration of Drosophila.J Neurosci. 2012 Aug 1;32(31):10758-66. doi: 10.1523/JNEUROSCI.0565-12.2012. J Neurosci. 2012. PMID: 22855823 Free PMC article.
-
The Role of Reversible Phosphorylation of Drosophila Rhodopsin.Int J Mol Sci. 2022 Nov 24;23(23):14674. doi: 10.3390/ijms232314674. Int J Mol Sci. 2022. PMID: 36499010 Free PMC article. Review.
-
β-arrestins and G protein-coupled receptor trafficking.Handb Exp Pharmacol. 2014;219:173-86. doi: 10.1007/978-3-642-41199-1_9. Handb Exp Pharmacol. 2014. PMID: 24292830 Free PMC article. Review.
Cited by
-
Proximity labeling reveals interactions necessary to maintain the distinct apical domains of Drosophila photoreceptors.J Cell Sci. 2024 Dec 1;137(23):jcs262223. doi: 10.1242/jcs.262223. Epub 2024 Dec 11. J Cell Sci. 2024. PMID: 39540276 Free PMC article.
-
Comprehensive study of SNAREs involved in the post-Golgi transport in Drosophila photoreceptors.Front Cell Dev Biol. 2024 Dec 10;12:1442192. doi: 10.3389/fcell.2024.1442192. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39720007 Free PMC article.
-
Differential activation of rhodopsin triggers distinct endocytic trafficking and recycling in vivo via differential phosphorylation.PLoS One. 2024 Jun 7;19(6):e0303882. doi: 10.1371/journal.pone.0303882. eCollection 2024. PLoS One. 2024. PMID: 38848405 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

