CD44-targeting hyaluronic acid-selenium nanoparticles boost functional recovery following spinal cord injury
- PMID: 38263204
- PMCID: PMC10804833
- DOI: 10.1186/s12951-024-02302-0
CD44-targeting hyaluronic acid-selenium nanoparticles boost functional recovery following spinal cord injury
Abstract
Background: Therapeutic strategies based on scavenging reactive oxygen species (ROS) and suppressing inflammatory cascades are effective in improving functional recovery after spinal cord injury (SCI). However, the lack of targeting nanoparticles (NPs) with powerful antioxidant and anti-inflammatory properties hampers the clinical translation of these strategies. Here, CD44-targeting hyaluronic acid-selenium (HA-Se) NPs were designed and prepared for scavenging ROS and suppressing inflammatory responses in the injured spinal cord, enhancing functional recovery.
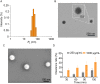
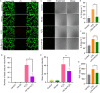
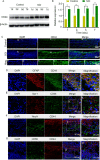
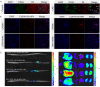
Results: The HA-Se NPs were easily prepared through direct reduction of seleninic acid in the presence of HA. The obtained HA-Se NPs exhibited a remarkable capacity to eliminate free radicals and CD44 receptor-facilitated internalization by astrocytes. Moreover, the HA-Se NPs effectively mitigated the secretion of proinflammatory cytokines (such as IL-1β, TNF-α, and IL-6) by microglia cells (BV2) upon lipopolysaccharide-induced inflammation. In vivo experiments confirmed that HA-Se NPs could effectively accumulate within the lesion site through CD44 targeting. As a result, HA-Se NPs demonstrated superior protection of axons and neurons within the injury site, leading to enhanced functional recovery in a rat model of SCI.
Conclusions: These results highlight the potential of CD44-targeting HA-Se NPs for SCI treatment.
Keywords: CD44 targeting; Inflammation; Reactive oxygen species; Selenium nanoparticles; Spinal cord injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Selenium-Doped Carbon Quantum Dots Efficiently Ameliorate Secondary Spinal Cord Injury via Scavenging Reactive Oxygen Species.Int J Nanomedicine. 2020 Dec 14;15:10113-10125. doi: 10.2147/IJN.S282985. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33363370 Free PMC article.
-
Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation.J Nanobiotechnology. 2024 Oct 24;22(1):653. doi: 10.1186/s12951-024-02916-4. J Nanobiotechnology. 2024. PMID: 39443923 Free PMC article.
-
Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model.J Control Release. 2023 Dec;364:109-123. doi: 10.1016/j.jconrel.2023.10.028. Epub 2023 Oct 27. J Control Release. 2023. PMID: 37866402 Free PMC article.
-
CD44-specific nanoparticles for redox-triggered reactive oxygen species production and doxorubicin release.Acta Biomater. 2016 Apr 15;35:280-92. doi: 10.1016/j.actbio.2016.02.005. Epub 2016 Feb 4. Acta Biomater. 2016. PMID: 26853764
-
The role of timing in the treatment of spinal cord injury.Biomed Pharmacother. 2017 Aug;92:128-139. doi: 10.1016/j.biopha.2017.05.048. Epub 2017 May 20. Biomed Pharmacother. 2017. PMID: 28535416 Review.
Cited by
-
Spinal cord injury repair based on drug and cell delivery: From remodeling microenvironment to relay connection formation.Mater Today Bio. 2025 Feb 4;31:101556. doi: 10.1016/j.mtbio.2025.101556. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40026622 Free PMC article. Review.
-
Integrative analysis and experimental validation identify the role of CD44 and Nucleolin in regulating gliogenesis following spinal cord injury.Cell Regen. 2025 Aug 13;14(1):35. doi: 10.1186/s13619-025-00253-x. Cell Regen. 2025. PMID: 40797111 Free PMC article.
-
KLK8/HGF/Met signaling pathway mediates diabetes-associated hippocampal neuroinflammation in male mice.Theranostics. 2025 May 25;15(13):6290-6312. doi: 10.7150/thno.109513. eCollection 2025. Theranostics. 2025. PMID: 40521191 Free PMC article.
-
Targeted delivery of HSP90 inhibitors for efficient therapy of CD44-positive acute myeloid leukemia and solid tumor-colon cancer.J Nanobiotechnology. 2024 Apr 22;22(1):198. doi: 10.1186/s12951-024-02460-1. J Nanobiotechnology. 2024. PMID: 38649957 Free PMC article.
-
Biomaterials targeting the microenvironment for spinal cord injury repair: progression and perspectives.Front Cell Neurosci. 2024 May 9;18:1362494. doi: 10.3389/fncel.2024.1362494. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38784712 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

