Interference of pseudorabies virus infection on functions of porcine granulosa cells via apoptosis modulated by MAPK signaling pathways
- PMID: 38263223
- PMCID: PMC10807058
- DOI: 10.1186/s12985-024-02289-y
Interference of pseudorabies virus infection on functions of porcine granulosa cells via apoptosis modulated by MAPK signaling pathways
Abstract
Background: Pseudorabies virus (PRV) is one of the major viral pathogens leading to reproductive disorders in swine. However, little is known about the effects of PRV infection on porcine reproductive system. Ovarian granulosa cells are somatic cells surrounding oocytes in ovary and required for folliculogenesis. The present study aimed to investigate the interference of PRV on functions of porcine ovarian granulosa cells in vitro.
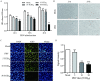
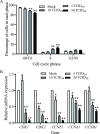
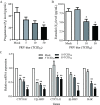
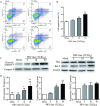
Methods: Primary granulosa cells were isolated from porcine ovaries. To investigate the PRV infectivity, transmission electron microscopy (TEM) was used to check the presence of viral particles, and the expression of viral gE gene was detected by quantitative real-time PCR (qPCR) in PRV-inoculated cells. After PRV infection, cell viability was detected by MTS assay, Ki67 for proliferative status was determined by immunofluorescence assay (IFA), cell cycle and apoptosis were detected by flow cytometry, and progesterone (P4) and estradiol (E2) were determined by radioimmunoassay. The checkpoint genes of cell cycle and apoptosis-related proteins were studied by qPCR and western blotting.
Results: Virus particles were observed in the nucleus and cytoplasm of PRV-infected granulosa cells by TEM imaging, and the expression of viral gE gene increased in a time-dependent manner post infection. PRV infection inhibited cell viability and blocked cell cycle at S phase in porcine granulosa cells, accompanied by decreases in expression of Ki67 protein and checkpoint genes related to S phase. Radioimmunoassay revealed decreased levels in P4 and E2, and the expressions of key steroidogenic enzymes were also down-regulated post PRV-infection. In addition, PRV induced apoptosis with an increase in Bax expression and activation of caspase 9, and the phosphorylation of JNK, ERK and p38 MAPKs were significantly up-regulated in porcine ovarian granulosa cells post PRV infection.
Conclusions: The data indicate that PRV causes infection on porcine ovarian granulosa cells and interferes the cell functions through apoptosis, and the MAPK signaling pathway is involved in the viral pathogenesis.
Keywords: Apoptosis; MAPK signaling pathway; Ovarian granulosa cells; Pseudorabies virus; Steroidogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Transcriptomic analysis of the dialogue between Pseudorabies virus and porcine epithelial cells during infection.BMC Genomics. 2008 Mar 10;9:123. doi: 10.1186/1471-2164-9-123. BMC Genomics. 2008. PMID: 18331636 Free PMC article.
-
TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling.Virology. 2008 Nov 10;381(1):55-66. doi: 10.1016/j.virol.2008.08.023. Epub 2008 Sep 17. Virology. 2008. PMID: 18799179
-
Knockdown of CEBPβ by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis.J Steroid Biochem Mol Biol. 2014 Sep;143:90-8. doi: 10.1016/j.jsbmb.2014.02.013. Epub 2014 Mar 4. J Steroid Biochem Mol Biol. 2014. PMID: 24607812
-
The Attenuated Pseudorabies Virus Vaccine Strain Bartha Hyperactivates Plasmacytoid Dendritic Cells by Generating Large Amounts of Cell-Free Virus in Infected Epithelial Cells.J Virol. 2022 Jun 22;96(12):e0219921. doi: 10.1128/jvi.02199-21. Epub 2022 May 23. J Virol. 2022. PMID: 35604216 Free PMC article.
-
How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis.Int J Mol Sci. 2025 Apr 9;26(8):3542. doi: 10.3390/ijms26083542. Int J Mol Sci. 2025. PMID: 40332048 Free PMC article. Review.
Cited by
-
Pyrogallol protects against influenza A virus-triggered lethal lung injury by activating the Nrf2-PPAR-γ-HO-1 signaling axis.MedComm (2020). 2024 Apr 12;5(4):e531. doi: 10.1002/mco2.531. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38617435 Free PMC article.
-
LncRNA NORFA promotes the synthesis of estradiol and inhibits the apoptosis of sow ovarian granulosa cells through SF-1/CYP11A1 axis.Biol Direct. 2024 Nov 10;19(1):107. doi: 10.1186/s13062-024-00563-1. Biol Direct. 2024. PMID: 39523350 Free PMC article.
-
The roles of MAPK signaling pathway in ovarian folliculogenesis.J Ovarian Res. 2025 Jul 14;18(1):152. doi: 10.1186/s13048-025-01737-9. J Ovarian Res. 2025. PMID: 40660328 Free PMC article. Review.
-
Intrauterine inoculation of pseudorabies virus impairs mouse embryo implantation via inducing inflammation and apoptosis in endometrium.Front Vet Sci. 2024 Oct 31;11:1475400. doi: 10.3389/fvets.2024.1475400. eCollection 2024. Front Vet Sci. 2024. PMID: 39545261 Free PMC article.
References
-
- Mettenleiter TC. Aujeszky’s disease (pseudorabies) virus: the virus and molecular pathogenesis-state of the art, June 1999. Vet Res. 2000;31:99–115. - PubMed
-
- Kimman TG, De Wind N, De Bruin T, de Visser Y, Voermans J. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology. 1994;205(2):511–8. doi: 10.1006/viro.1994.1672. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

